|
VARIABILIDAD ESPACIAL Y ESTACIONAL DE LA BIOMASA Y LA
MORFOLOGíA DE LAS HOJAS DEL PASTO MARINO Syringodiam filiforme, EN UNA
LAGUNA ARRECIFAL TROPICAL EN MÉXICO
Trabajo recibido el 15 de
octubre de 1992 y aceptado para su publicación el 25 de marzo de
1993.
Brigitta. L van Tussenbroek
Estación Puerto Morelos, Instituto de
Ciencias del Mar y Limnología. Apartado Postal 145, 77500, Q. Roo,
MÉXICO. Contribución No. 741 del Instituto de Ciencias del Mar y
Limnología.
Se tomaron muestras de pasto marino
Syringodium filiforme, mediante un nucleador, a intervalos
mensuales o bimestrales de julio de 1990 hasta noviembre de 1991, en tres
estaciones de la laguna arrecifal de Puerto Morelos, México. Se
registraron diferencias en biomása total, la distribución de
biomása de varias partes de la planta y la morfología de las
hojas en las estaciones de muestreo. Estas diferencias locales fueron debidas
posiblemente a la distribución de la abundancia de pasto
Thalassia testudinum en esos lugares. También se
registraron variaciones estacionales de la biomása de las distintas
estructuras de la planta. La biomása total varió de 959.2 a 1,
158.6 g peso seco · m-² en una franja costera monoespecífica
de S. filiforme. La biomása total en una
estación costera y en una lagunar donde dominaba T.
testudinum varió de 183.9 a 288.1 g peso seco ·
m-² y de 89.9 a 127.8 g peso seco · m-², respectivamente. La
biomása máxima fue registrada durante el verano y los valores
mínimos en invierno. La longitud y el diámetro de las hojas
fluctuaban similarmente de acuerdo con las estaciones durante el período
de estudio, y se encontró una correlación entre estos
parámetros morfológicos y la biomása de las hojas. Las
estructuras reproductivas fueron encontradas desde enero hasta el verano en las
estaciones costeras. Sin embargo, su aportación a la biomása
total fue insignificante
PALABRAS-CLAVE:
Syringodium, biomása, morfología de las hojas,
variaciones estacionales.
Corer samples were taken of the seagrass
Syringodim filiforme at monthly or binionthly intervals from
July 1990 until November 1991, at three sampling stations in a tropical coral
reef lagoon, Mexico. Total biomass distribution and leaf morpliology varied
between the sampling stations, which was attributed to competition with
Thalassia testudinum at those stations. At the three
sampling stations, seasonal variance in biomass of aboye and 2 below ground
parts were recorded. Total biomass varied between 959.2 and 1,158.6 g dry
weight · m-² at a virtually monospecific coastal fringe of
S. filiforme, between 183.9 and 288.1 g dry weight m-²
at a costal station and between 89.9 and 127.8 g dry weight · m-²
at a midlagoon station. T. testidinum dominated the
vegetation at the latter two stations. biomass was minimuni during the winter
and maximum in the summer montlis. These seasonal changes were likely due to
changes in water temperature as is suggested by significant correlations
between total biomass and temperature. Leaf length and leaf diameter also
varied with season and were correlated to fluctuations in leaf biomass.
Inflorescences were recorded from January until the summer at the two coast
stations. However, inflorescence biomass never accounted for more than 12.1 %
of the total above-ground biomass.
KEY WORDS: Syringodium,
biomass, leaf morphology, seasonal variations.
Seagrass beds from the Caribbean and the Gulf of Mexico, are characterized by the coexistance of the turtle grass Thalassia testudinum and the manatee grass Syringodium filiforme. Observations on T. testudinum biomass are numerous (McRoy and McMillan, 1977; Zieman and Wetzel, 1980 for sumaries), whereas S. filiforme has recieved limited attention. So reported Zieman and Wetzel (1980) that "in the semitropics, 73 % of biomass and productivity measurements were for T. testudinum". This relatively large interest for T. testudinum in comparison with other tropical seagrasses might partly be explained by this seagrass species being the dominant species of Caribean seagrass communities (Zieman, 1982). In the Caribbean, monospecific stands of S. filiforme are considered to be an intermediate stage in the succession of a virgin area, facilitating colonization of the chmax species T. testudinum (Patriquin, 1975). In early succession models S. filiforme was completely replaced by T. testudinum (Zieman, 1982), however, Williaras (1990) found that "co-existance, rather than replacement, of species occurs". In the subtropical area of Florida, Syringodium filiforme undergoes seasonal variations in leaf biomass (Gilbert and Clark, 1981), primary production (Barber and Behrens, 1985), leaf morphology (Strawn, 1961), and levels of proximate constituants of the rhizomes (Dawes and Lawrence, 1980). In tropical Cuba, Buesa (1975) did not record seasonal variations in S. filiforme biomass. However, he had only sampled four times in a year, which could be insufficient for following trends in tropical areas. Seasonal changes in seagrass biomass are generally less pronounced in tropical areas than in temperate waters (Duarte, 1989; Hillman et al., 1989), thus a regular sampling scheme is needed to observe possible seasonal fluctuatios. The aim of the present study is to determine whether, besides local variations, seasonal fluctuations in S. filiforme biomass and leaf morphology occur in a tropical reef lagoon in Mexico. STUDY AREAThe Puerto Morelos reef lagoon, Mexico (20° 5 ' N, 86° 55' W), is delimited by a fringing reef situated ca. 550m to 1.5 km from the coastline. Environmental conditions are principally determined by marine influence, as terrestrial runoff is negligible due to the scarcity of soil upon the karstic continental terrain. This is reflected in a stable water salinity (mean 35.7% ± 9.7 S.D.) and low mean nitrite (0.06 µg-at N1-¹), nitrate (13.9 µg-at N1-¹) and phosphate (0.46 µg-at P1-¹) concentrations (Merino and Otero, 1991). Winds mainly blow from South to Southeast; strong northem winds disturb these prevailing trade winds during the study winter months. Water temperature record during the period is pictured in Figure 1. Substratum consists of coarse carbonate sand. Two coastal stations, at a depth of ca. 2.5 m, were elected near the marine research station of the National University of Mexico. The fírst station (Coast-1), is virtually a monospecific fringe of the manatee grass Syringodium filiforme with very little Thalassia testudinum. At the second station (Coast-2), the turtle grass T. testudinum dominates the other components of the vegetation being S. filiforme, rhizophytic algae, Halimeda spp. and Avrainvillea spp. A third mid- lagoon sampling station near Punta Caracol (Lagoon), at a depth of ca. 5 m, is characterized by a lush growth of T. testudinum accompanied in low densities by S. filiforme, rhizophytic algae, Halimeda spp. and Avrainvillea spp. MATERIAL AND METHODS
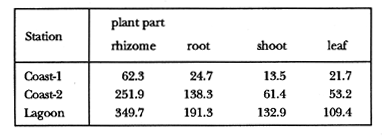 TABLE 1 Thalassia testudinum biomass ( dry g m-2 ) at the three sampling in the Puerto Morelos reef lagoon. Data from samples collected in July 1991. Two corer samples (diam. 20 cm, depth 30 to 35 cm) were taken at montly or bimontly intervals per station from July 1990 until November 1991. Sed¡ment was removed and Syringodium filiforme was sorted from the samples. The plants were separated in the following aboveground parts: leaves and inflorescences, and belowground parts: live rhizomes, live roots and live short-shoots. Short-shoots bearing the leaves and inflorescences were placed in a 10% Phosphoric acid solution and rinsed with fresh water to remove calcareous epiphytes. Total number of leaves were counted for each corer sample. Length and diameter were determined for the ten largest leaves per corer sample, if sample size was not sufficiently large to contain 10 large leaves (i.e. more than 10 cm long), a smaller number of leaves were measured. The plant parts were dried at 60°C during 24 hours and weighed afterwards. Due to the uneven distribution of S. filiforme, the number of leaves varied considerably between corer samples (Table 2). It was assumed that the leaf density of S. filiforme was independent of the time of the year. The values for plant parts dry weight of the corer samples at a station, were standarized by calculating the dry weight for samples having the mean number of leaves for all samples collected at that station (Table 2). This calculated dry weight was summed for the 2 corer samples taken at one time at each station, and converted to dry weight per m2 to obtain a value for the biomass of the plant parts. 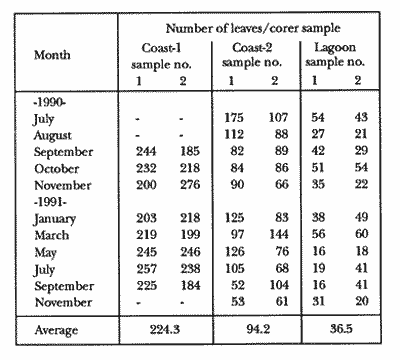 TABLA 2 Syringodium filiforme number of leaves of two corer samples (diameter 22 cm ) at the three station in the Puerto Morelos reef lagoon. Average is for all samples taken at a certain station (- no samples taken ) 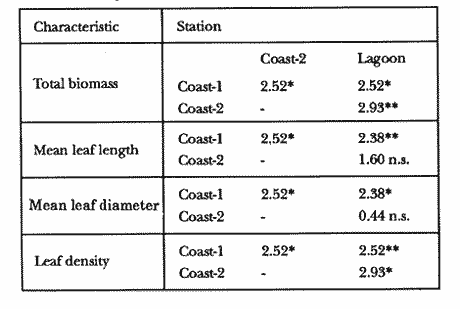 TABLE 3 Results of the Wilcoxon Paired Sample Test (T) for the analysis of differences of Syringodium filiforme biomass and leaves characteristics between the sampling station at the Puerto Morelos reff lagoon. n=11 for Coast-2 and Lagoon comparisons, n=9 for Coast-1 comparisons. n.s. not significant, *p=0.05 **p=0.01. Differences in total biomass, leaf length, diameter and density between sampling stations were determined with the Wilcoxon Paired Sample Test. The Friedínan Analysis of Variance by Blocks was used to test whether the biomass of the plant parts varied between sampling times per station; the values for the biomass of the plant parts were considered as blocks. To determine whether the biomass proportion of the plant parts varied throughout the sampling time per station, the G-test of Independance was used. Differences in leaf length between the sampling times were tested with the One way Analysis of Variance. Product Moment Correlations were computed between total biomass and mean water temperature and between leaf biomass and mean leaf length and diameter. 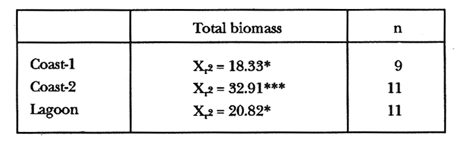 Table 4 Resulats of the Friedman Analysis of Variance by blocks testing variations in biomass with sampling time at the three sampling station in the Puerto Morelos reef lagoon. *p=0.05, **p=0.01, ***p=0.001 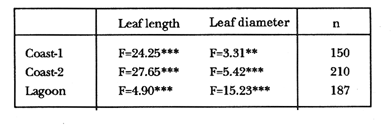 Table 5 Results of the Oneway Analysis of Variance testing variations in leaf length and diameter per sampling in the Puerto Morelos reef lagoon. *p=0.05, **p=0.01, ***p=0.001 RESULTSData on T. testudinum biomass at the three sampling stations are presented in table 1. Syringodium filiforme total biomass, leaf length, leaf diarneter and leaf density differed significantly between the stations, except for the leaves at the Coast-2 and Lagoon station which had a similar length and diameter (Table 3). Leaves accounted for a similar porportion of the total biomass at all three stations whereas the biomass distribution over the below-ground plant parts differed between the stations: (Fig. 2). 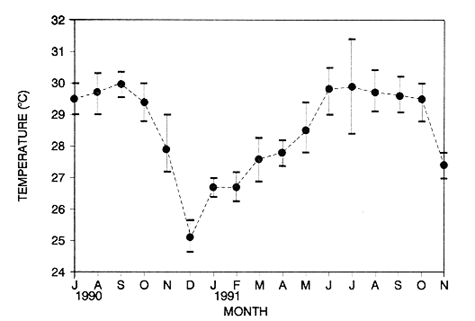 Figure 1. Mean temperature (+ S.D.) recorder 3 to 5 times a month in the Puerto Morelos reef lagoon during late-afternoon. Measurements for July, August and September 1990 were taken late morning (data from J.N. Alvares-Cadena ). 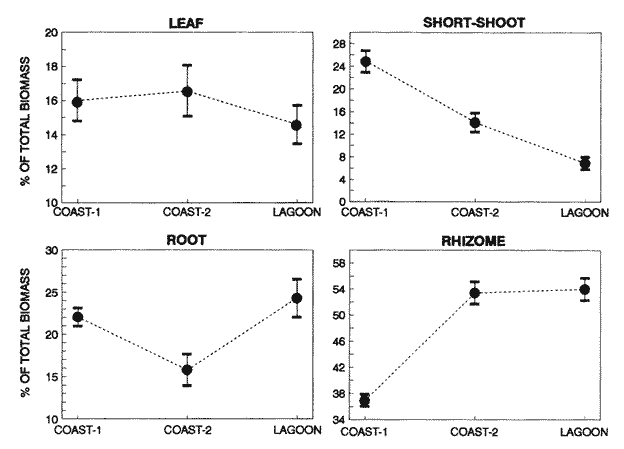 Figure 2. Mean percentage (+ 95% confidence limits ) of the total biomass of the S. filiforme plant parts for all samples collected from July 1990 until Novemver 1991, at the three sampling stations in the Puerto Morelos reef lagoon. Syringodium filiforme biomass varied significantly between sampling times (Table 4) and was minimum in the winter months and maximum during the summer or early autumn at all sampling stations(Fig. 3). However, no significant seasonal differences in the distribution of the biomass over the plant parts were found (G = 35.00, p = 0.05 for Coast-1, G = 35.09, p = 0.01 for Coast-2 and G = 11.97, p = 0.001 for Lagoon station). Leaf length and diameter also varied significantly between the sampling intervals (Table 5) and showed similar seasonal trends as biomass (Fig. 4). Significant correlations were found between leaf biomass and leaf morphology (Table 6). 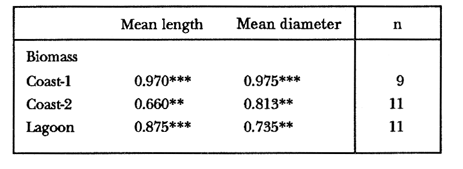 Table 6 Product Moment Correlation Coefficients (r) between Syringodium filiforme leaf biomass and mean leaf length and diameter for the three sampling stations in the Puerto Morelos reef lagoon. **p=0.01, ***p=0.001. Biomass was correlated with mean water temperature and the correlation coefficients were 0.94 (p = 0.01), 0.88 (p = 0.001) and 0.69 (p = 0.05) for the Coast-1, Coast-2 and the Lagoon stations respectively. Inflorescences were recorded from January until July at the Coast-1 station and from January until May at the Coast-2 station; at the Lagoon station no inflorescences were recorded during the study pe- riod. Inflorescences biomass was largest at Coast-1 station, however, it never reached more than 12.14% of the total above-ground biomass (Table 7). 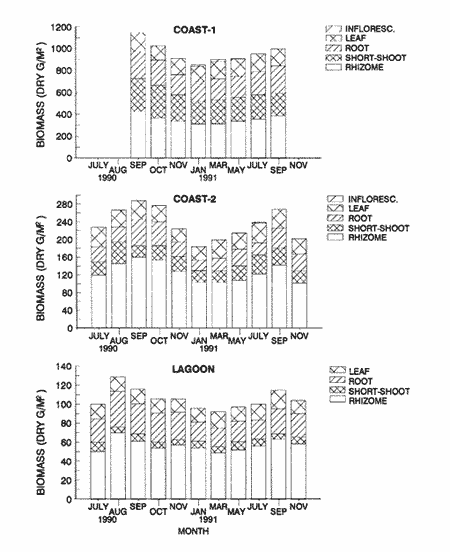 Figure 3. Biomass of Syringodium filiforme plant parts recorded from July 1990 until November 1991 at Coast-1, Coast-2 and Pta. Caracol in the Puerto Morelos reef lagoon. DISCUSSIONDifferences of Syringodium filiforme characteristics between the three sampling stations could possibly be attributed to competion with the turtle grass Thalassia testudinum. Williams (1987) found that removal of T. testudinum leaves resulted in an increase of S. filiforme shoot-density and above-and below-ground biomass. In the present study, leafdensity, above-and belowground biomass were lowest at the Lagoon station where biomass of turtle grass was highest and lowest at the Coast-1 station, where the turtle grass was virtually absent (Table 1). At the Coast-1 station. S. filiforme rhizomes were found until a depth of ca. 20 to 25 cm whereas at the other two stations rhizomes of this species were found above the T. testudinum rhizomes at a depth varying between 2 to 5 cm. Consequently, the shortshoots at the Coast-1 station were longer. This resulted in a proportionally higher short-shoot biomass and lower rhizome biomass at the Coast-1 station in comparison with the other two stations. The leaves under a T. testudinum canopy at the Coast-2 station, where shorter and thinner than at the virtually monospecific stand at the Coast-1 station. These differences in leaf morphology between these stations were possibly due to shading of T. testudinum, with a mean leaf width of 1 cm, has a much greater leaf area and thus intercepts more light than S. filiforme. Explanation for the differences in the proportion of aboveground biomass occupied by inflorecences at the two coastal stations could also possibly be attributed to the increased shading by T. testudinum leaves at the Coast-2 station. The absence of reproductive structures at the Lagoon station, was most likely due to the low S. filiforme density. The maximum number of leaves per sample at this station was 60 with a mean of 36.5. Whereas at the Coast-2 station, at the peak of flowering, only one flowering shoot per 80 leaves was observed. 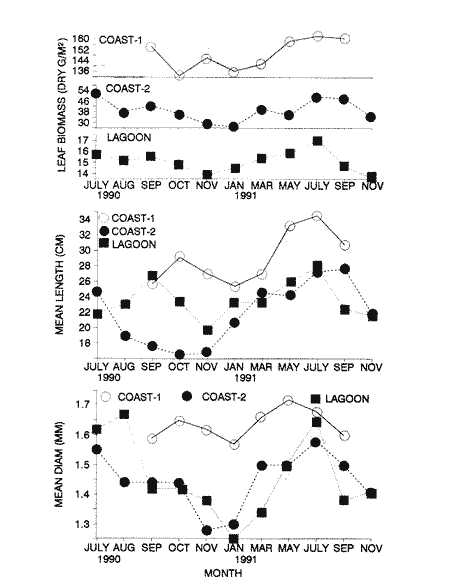 Figure 4. Syringodium filiforme leaf biomass (note differing ordinate inean scales for the stations), mean leaf length and mean leaf diameter recorded from July 1990 until November 1991 at the three sampling stations in the Puerto Morelos reef lagoon. Dashed thin lines indicate 95% confidence limits. Seasonal variance of Syringodium filiforme biomass at the Puerto Morelos reef lagoon was small in comparison with that recorded for Central Florida, where Gilbert and Clark (1981) found the leaf biomass to vary between 5 and 42 g dry weight o m7 2. The population studied by Gilbert and Clark (1981) was situated near the nothem distribution limit of this seagrass species which might well account for their large fluctuations. This confírins the results of the analysis of Duarte (1989) that the biomass-variability increases with inceasing latitude, which according to Kain (1989) can most likely be attributed to greater environmental variability (especially light) at higher latitudes. 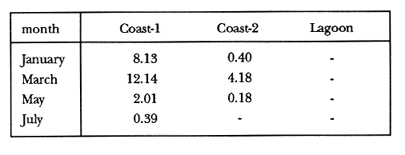 Table 7 Syringodium filifome percentage inflorescences biomass of total above ground biomass (i. e. leaves and inflorescences) during 1991 at the three sampling stations in the Puerto Morelos reef lagoon (-no inflorescence recorded) Temperature could well be responsible for the seasonal biomass fluctuations reported in this study, as is suggested by the significant correlation between temperature and total biomass. However, the influence of the amount of light can not be excluded, as the effects of light and temperature are difficult to separate in the field. Gilbert and Clark (1981) suggested that temperature, besides storms and burrying of the horseshoe crab, was one of the principal factors causing large biomass fluctuations. A more convincing evidence for the temperature dependence of S. filiforme net leaf productivity was presented by Barber and Behrens (1985), who studied this species in a sub-tropical lagoon in Florida, which received thermal addition from a thermal power plant. The seasonal pattern recorded for inflorescence biomass during the present study, confirín the fíndings of Mc Millan (1980) that Mowering occurs primarily under lengthening daylengths and warming temperatures that follow winter minima". At all three sampling stations, not only the aboveground biomass (Le. leaves and inflorescences) varied with season but also the belowground biomass. It is striking, that the belowground parts (especially the rhizomes) show seasonal variation, which suggests a very high turnover rate of these plant parts. An interesting observation is that in March, rhizome biomass had not increased substantially since January whilst the leaves showed a biomass increase. This suggests that after a winter minimum, most of the plants enery is directed towards leaf growth, and that the rhizome growth lags behind. Dawes and Lawrence (1980), who found that the soluble carbohydrate level in the rhizomes of 3 seagrass species increased during the summer in Florida, whereas in the winter and spring rhizome carbohydrates were low. They suggested that in winter and spring these carbohydrates were used for plant maintenance and growth, and that in the summer levels were high due to production and storage of starch. This would be reflected in different proportional biomass of leaves and rhizomes during winter and summer. Syringodium filiforme leaf morphology in the Puerto Morelos reef lagoon also showed seasonal variation. As the samples were standarized, having a similar number of leaves at all times, seasonal changes in leaf biomass can be attributed to changes in leaf morphology. This is confírmed by the positive correlations between leaf biomass with leaf length and diameter. AgradecimientosACKOWLEDGEMENTSI would like to take advantage of this opportunity to thank all those people at the Station of Puerto de Morelos who have helped me at some stage, to realize this work. Special thanks go to M. en C. Laura Celis Gutierrez, who helped to initiate this study,and to students who have accompanied me during the fieldwork. I am also gratefull to the collegues who revised the work and I would like to specially thank Dr. Jordan Dahlgren whose comments substantially improved the manuscript. LITERATURACITED LITTERATURE BARBER, B.J. and P.J. BEHRENS, Aquat. Bot. Effects of elevated temperature on seasonal in situ leaf productivity of Thalassia testudinum Banks ex Konig and Syringodium filiforme Kutzíng. 1985 61-69. 22 BUESA, R.J., Aquat. Bot. Populañons biomass and metabolic rates on marine angiosperms on the northwestem Cuban Shelf. 1975 11-23 1 DAWES, C.J. and J.M. LAWRENCE, Aquat. Bot. Seasonal changes in the proximate constituants of the seagrasses Thalassia testudinum, Halodule wrightú, and Syringodium filiforme 1980. 371-380 8 DUARTE, C.M., Mar. Ecol. Progr. Ser., 51 Temporal biomass variability and productionibiomass relationships of seagrass communities. 1989. 269-276. GILBERT, S. and K.B. CLARK, Estuaries Seasonal variation in standing crop of the seagrass Syringodium filiforme and associated macrophytes in the northern Indian River, Florida. 1981. 223-225. 4 HILLMAN, K., D.I. WALKEI, A.W.D. LARKUM and A.J. McCOMB Biology of seagrasses. Productivity and nutrient limitation: 635685.A treatise on the biology of seagrasses with special reference to the Australian region. In: A.W.D. Larkuín, A.J. McComb, S.A. Shepherd (Eds.). Elsevier Science Publishers B.V., Amsterdam. 1989 KAIN, J.M., The seasons in the subtidal Br. Phycol, J. 1989. 203-215. 24 McMILLAN, C., Amer, J. Bot. Reproductive physiology of the seagrass Syringodium filiforme, from the Gulf of Mexico and the Caribbean. 1980. 104-110 67 McROY, C.P. and C. McMILLAN, Seagrass ecosystems Production ecology and physiology of seagrasses In: C.P. McRoy and C. Helfferich (Eds.). A scientific perspective. marcel Dekker Inc., New York. 1977. 53-87 MERINO, M. and L. OTERO, Atlas ambiental costero, Puerto Morelos - Quintana Roo. Centro de Investigaciones de Quintana Roo. Centro de Investigaciones de Quintana Roo, Chetumal, México. 1991. 80 p. PATRIQUIN, D.G. Aquat. Bot. "Migration" of blowouts in seagrass beds at Barbados and Carriacou, West Indies, and its ecological and geological implications 1975. 163-189 1 STRAWN, K., J. Wildl. Manage. Factors Influencing the zonation of subinersed monocotyledons at Cedar key, Florida. 1961. 178-189. 25 WILLIAMS, S.L. Mar. Ecol. Progr. Ser. Competition between the seagrasses Thalassia testudinum and Syringodium fíliforme in a Caribbean lagoon. 1987. 91-98. 35 WILLIAMS, S.L., Ecol. Monogr., Experimental studied of Caribbean seagrass bed development. 1990. 449-469. 60 ZIEMAN, J.C. The ecology of the seagrasses of South Florida: A community profíle. U.S. Fish and Wildlife Services, Office of Biological Service, FWS/CBS82185. Washington, D.C. 1982 P 158. ZIEMAN, J.C. and R.O. WETZEL, Handbook of seagrass biology: An ecosytem perspective Productivity in seagrasses: methods and rates. In: R.C. Plifilips and C.P. McRoy (Eds.). Garland STPM Press New York 1980 87-116
|

