|
FEEDING HABITS, GONADIC STAGES AND SIZE
FREQUENCY DISTRIBUTION OF Sagitta setosa J. MÜLLER TO THE EAST OF THE
ISLE OF MAN, NORT IRISH SEA. PORT MARINE LABORATORY, PORT ERIN ISLE OF MAN,
U.K.
Trabajo recibido el 11 de agosto de 1992 y aceptado para su
publicación el 30 de octubre de 1992.
HÁBITOS ALIMENTARIOS, ESTADIOS GONÁDICOS Y DISTRIBUCIÓN DE FRECUENCIA DE TAMAÑOS DE Sagitta setosa J. MÜLLER AL ESTE DE LA ISLA DE MAN, NORTE DEL MAR DE IRLANDA. PORT ERIN MARINE LABORATORY, PORT ERIN ISLE OF MAN, U.K.
José N. Álvarez- Cadena
Estación Puerto Morelos, Instituto de Ciencias del Mar y
Limnología, UNAM. Apartado Postal 1152, Cancún 77500, Quintana
Roo, MÉXICO. Contribución No. 726 del Instituto de Ciencias del
Mar y Limnología.
Los
análisis del contenido digestivo en Sagitta setosa
indican que este quetognato se alimenta primordialmente de copépodos.
Oithona spp, Pseudocalanus elongatus y
Acartia clausi fueron las presas más frecuentemente
consumidas y fueron también las más abundantes en el área
de estudio. Otros zooplanctontes, como apendicularias y tintínidos,
también contribuyeron a la dieta de este depredador. La tasa de
alimentación varió de 1.38 a 5.35 presas por día. La
mayoría de los depredadores tenían una presa en el tracto
digestivo, aunque también se registraron presas múltiples. La
cantidad máxima de presas observadas en un depredador fue de nueve
(Stenosomella spp); sin embargo, la más frecuente fue
de dos. Aun cuando las colectas de zooplancton se realizaron mensualmente,
Sagitta setosa se registró únicamente desde
principios de otoño hasta finales del invierno. La reproducción
de la especie ocurrió en otoño, como se pudo comprobar por la
presencia de animales en estadio I con tallas menores a 3 mm. El desove
disminuyó a finales del otoño; en invierno no se encontraron
animales en estadio III de madurez gonádica. Se sugiere que, en el Mar
de Irlanda, Sagitta setosa tiene sólo una
generación anual.
Gut content analysis of
Sagitta setosa indicated that its diet consisted mainly of
copepods. Oithona spp, Pseudocalanus elongatus
and Acartia clausi were the main prey consumed
and were also found to be most abundant in the study area. Other zooplankters
such as appendicularians and tintinnids also contributed to the alimentation of
this chaetognath. The Feeding Rate (FR) was found to range from 1.38
prey-day-¹ to 5.35 prey-day-¹ Most predators were found having a
single prey in their gut. Maximum number of prey in a predator was 9
(Stenosomella spp); however, the most common multiple prey
was 2 prey items. Although zooplankton samples were collected monthly,
Sagitta setosa was only found from early autumn until late
winter. Spawning occurred in autumn as was shown by the presence of small stage
I animals less than 3 mm in length. Spawning activity decreased towards autumn
end, and no animals at the mature stage III were found in winter. It is
suggested that S. setosa in the Irish Sea has only one brood
per year.
Chaetognaths are strictly carnivorous (Alvariño, 1965), and are thought to be an important link in the energy conversion from primary producers, via copepods, into higher trophic levels (Reeve, 1970). Sagitta setosa and S. elegans are the most abundant chaetognaths in European (Oresland, 1987) and boreal waters (Alvariño, 1965). Both species have been regarded as indicators for characterizing water masses (Rusell, 1935, 1939; Williamson, 1956; Khan and Williamson, 1970). In British waters, S. setosa is usually common in bays and estuaries of the Welsh, English and Scottish coasts (c.f. Pierce, 1941; Williamson, 1956). S. elegans, on the other hand, is considered to be oceanic. Khan (1970), working in the Irish Sea, however, reported a changing overlap in the distribution of both species, which varied depending on the year's time. Pierce (1941) reported shifts in the autumn populations from S. elegans to S. setosa in Isle of Man waters. The biology of Sagitta elegans in the Irish Sea has been previously discussed (Álvarez-Cadena, in press). This work attempts to outline the life cycle of Sagitta setosa, based on the results found in the present study, combined with those of previous works. Data on food and feeding habits, gonadic stages and size frequency distribution of this species are also presented. MATERIAL AND METHODSPlankton collections were obtained from February 1986 until November 1987 to the East of the Isle of Man [latitude 54°03' N, longitude 04° 40' W (Fig. 1)]. Simultaneous horizontal tows were made at subsurface and near the bottom (34 m depth) with conical plankton nets, 1.30 m length and 0.46 m diameter (mesh size 0.330 mm), with General Oceanic flowmeters attached to the mouth. The nets were towed at 1.5 to 2 knots during 15 min. The samples were immediately fixed by adding concentrated buffered formaldehyde (CaCO3) solution until a final strength of ca. 5 % was obtained. Sampling was carried out every 3 hours in 1986 and every 2 hours in 1987 durig 12 hours cruises at day and at night, except in November both years, when only day samples were obtained. Temperature fluctuations at the sampling site are described in Graziono (1989), and salinity data in Slinn and Eastham (1984). 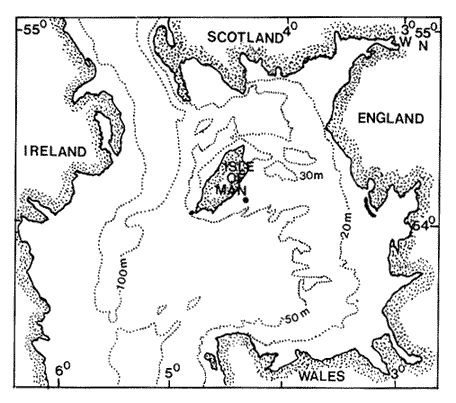 Figure 1. Sampling station in Isle of Man waters Depth profile, in meters. Specimens of Sagitta setosa containing food items were placed in a small tray, gut contents removed, and analyzed with a dissection microscope or, when higher magnification was requied with a non-stereo microscope, 400X, 600X. Chaetognaths are known to feed actively in the densely packed net can, during the sampling (Pearre, 1974). Thus, some extra feeding may have occurred before fixing the samples. Only those food items found in the posterior 1/3 of the gut and with some evidence of digestion were recorded. However, when the food items were small (e.g. tintinnids), they were recorded regardless of the position in the gut, as such small organisms probably would not be retained by the net with a mesh size, as described above, and the catch of this prey is likely to have occurred in nature. Identification of the food items was carried out following Giesbrecht (1892) and Rose (1933) identification keys for copepods, Dodge (1982) for dinoflagellates and Tregouboff and Rose (1957) for other zooplankters. Copepods are reported to be the most important prey for chaetognaths (Feigenbaum and Maris, 1984; Resland, 1987). As was found by Sullivan et al., (1975), heavily digested material can be determined to species by observing the cutting blades of the copepod's mandibles. In this study the most abundant copepods from the plankton samples were drawn, their mandibles dissected and mounted on permanent slides. The species concerned were: Calanus spp (copepodite II, IV and adult), Temora longicornis (copepodite II and adult), Centropages spp (copepodite III and adult), Acartia clausi (copepodite III and adult), Pseudocalanus elongatus (copepodite III and adult), and Oithona spp (adult). This allowed the identification of most food items. Appendicularians could be determined by means, of observing their faecal pellets as they resist digestion (Shelbourne, 1962). In assessing the feeding behaviour of Sagitta setosa, the Food Containing Ratio (FCR) was calculated following Feigenbaum. and Maris (1984) methods. Feeding Rate (FR) was determined by using the Bajkov's equation (1935): 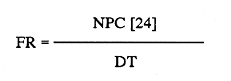 where NPC (multiple prey) is the mean Number of Prey per Chaetognath, DT is Digestion Time in hours, and 24 is a day-night cycle. Digestion time was calculated by using the formula DT = 3.34e-0.117t, developed by Mironov (1960). For the measurements and gonadic stages of the animals 50 to 100 specimemn of Sagitta setosa were randomly drawn These methods are described in Álvarez-Cadena (in press). RESULTS
FEEDINGZooplankton assemblages in both years were dominated mainly by Pseudocalanus elongatus and Acartia clausi. Appendicularians were only relatively important in the samples in 1987 (Fig. 2). 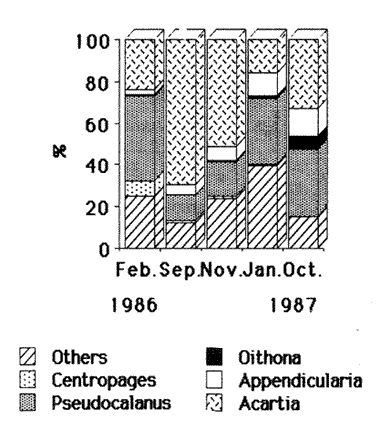 Figure 2. Compositon of the main zooplankters during 1986-1987; figures were obtained from 24 hours surveys, except for November 1986, when only day samples were taken. In February 1986, Sagitta setosa was mainly preying upon Oithona (28.6 %), Pseudocalanus (22%) and appendicularians (15.4%) (Fig. 3). Sagitta spp, contributed with 1.8% to the diet and food items as small as Lamellibranchia and Coscinodiscus were recorded, although in low percentages. In September of the same year, S. setosa was feeding mainly on appendicularians (29%) and 3 species of copepods, namely Acartia (23%), Pseudocalanus(20%) and Oithona (10%). November showed a similar pattern for the gut content analysis, including a 6% Dynophysis contribution to the prey eaten (Fig. 3). In January 1987, when the species was collected again, food mainly consisted of appendicularians (41%), Nauplii (12%) Pseudocalanus (9.6%) and Oithona (7.4%). Herring was also preyed upon more heavily than before (6%). In October, appendicularians and the tintinnid Stenosomella contributed significantly (27.7% and 24.3%, respectively), and Pseudocalanus, Oithona and Acartia were the other important prey in the diet components (Fig. 3). 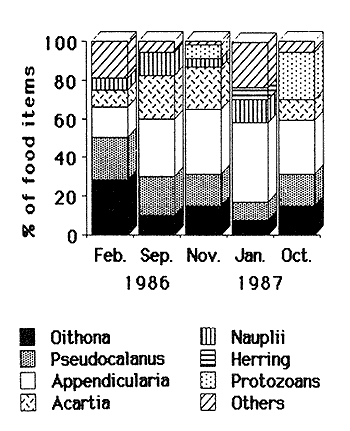 Figure 3. Percentage of the main food items recorded in the gut of Sagitta setosa during1986-1987 Feeding Rate (FR) for Sagitta setosa ranged from 1.38 to 5.35 prey day-¹ (Table 1). Feeding was consistently higher at night than during the day; however, when trying to determine if feeding was higher near surfase or near bottom, the FCR did not show a definite pattern (Table 1). 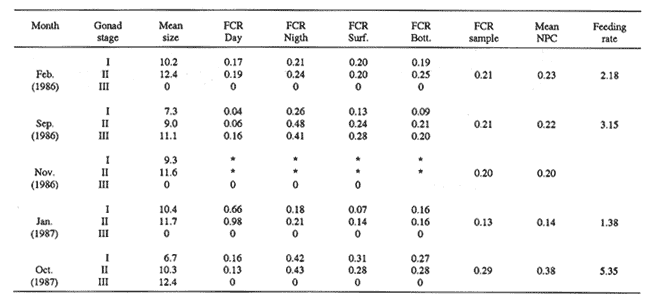 TABLE 1. Food Conntaining Ratio (FCR), Numberof Prey per Chaetognath (NPC) and Feeding Rate in Sagitta setosa at the sampled site in 1986 and 1987.* Not enough data. POPULATIONSSagitta setosa was found from late summer to late winter in the sampled site. It was, however, abundant only in September 1986 and October-November 1987. Due to this seasonality and also to other factors such as currents, the size frequency and abundance assesment are difficult (see discussion below). In September 1986, the mean size of the population was 8.6 mm. and increased to 10.5 mm by November of the same year. A further increment to 11.5 mm. was recorded in January 1987 (Fig. 4). In October 1987, the species was collected again; at that time the mean size of the population was 8.8 mm; in November it was 7.8 min. Sagitta setosa was first recorded in the samples collected in February 1986. At that time the smallest specimens measured 5.8 mm, whereas the largest ones were 14.8 mm long. Stage I dominated the population, contributing 83%, the remaining 17% were animals in stage II; no stage III individuals were recorded (Fig. 4). The following months this species dissapeared from the area, and was not recorded until September, when spawning had already started, as was evident by the presence of the three maturity stages. That month, animals at stage I (38%) had a minimum size of 4.4 mm (mean size for stage I was 6.6 mm), suggesting that spawning had probably started the previous month (see discussion below). Thirty three percent of the population was at stage II and 28% was at stage III, thus there was, no clear dominance of any stage in this month. Due to bad weather and ship access, no samples were collected in October of that year. November was characterized by the dominance of animals at stage I (75%), which had increased in mean size (9.8 mm), while specimens at stageIII had practically dissapeared (ca. 2%), indicating that breeding had nearly been concluded. 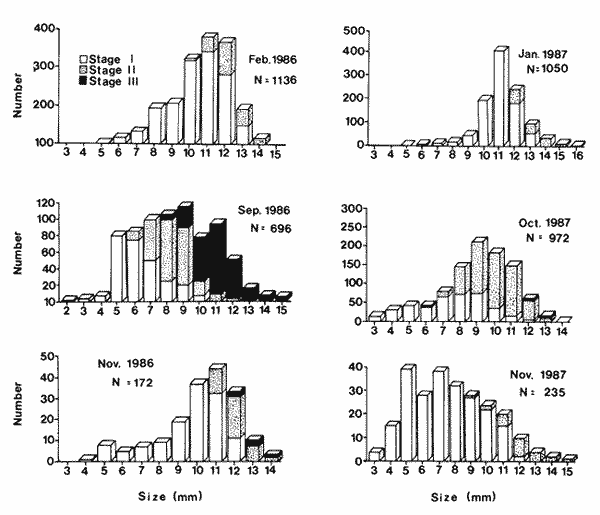 Figure 4. Size frequency distribution and gonadic stages of Sagitta setosa during 1986-1987. Sagitta setosa overwintered mainly as gonadic stage I individuals. In January 1987, animals in stage I had increased in dominance (87%) and also in mean size (11.2 mm). Specimens at stage II contributed with the remaining 13%, and no stage III animals were recorded (Fig. 4). As the preceding year, S. setosa dissapeared from the area the following months. No animals of this species were caught in September 1987, as samples in that month were only obtained just offshore of Port St. Mary, and the species was probably present further east. This assumption seems justified as in October the population mainly consisted of stages I and II (40% and 59%, respectively). The mean size of stage I animals (6.70 mm) indicated that spawning had started several weeks before, while the scarcity of stage III individuals (1%) indicated that breeding was nearly completed (Fig, 4). The next month, stage I was more dominant (94%), and the mean population si ze had increased to 7.6 mm. The remaining 6% consisted of stage II; no stage III animals were recorded in the population. DISCUSSIONSagitta setosa did not feed on organisms that were always representatively caught by the net. Consequently, the relationship between composition and abundance of the zooplankton recorded in the study area with the gut content of this species can be only partially done. The copepods Pseudocalanus, Acartia and Oithona were the main components in the Sagitta setosa alimentation. Pseudocalanus and Acartia were also abundant in plankton samples; however, Oithona was not found to be abundant in the study area. Specimens of this genus might have escaped through the net as the mesh size used was 0.33 mm, and Lee (1971) found that Oithona similis outnumbered all the other copepod species. Other prey items such as appendicularians and tintinnids were consistently recorded in the gut content of small sagittas. They, as in the case of Oithona, were probably underestimated in the samples. Graziano (1989) reported a high abundance of Stenosomella spp; these oligotrichids were numerically abundant in October 1987, and contributed significantly to the diet of S. setosa at that time. Thus it is very well possible that the gut contents of Sagitta setosa did generally reflect the zooplankton composition and abundance of the studied area. Results obtained in this study concerning size frequency and gonadic stages agree with those from Russell (1932, his plate II), Pierce (1941, his Fig. 2), Jakobsen (1971,his Figs. 14-15), and O'Brien (1976, his Fig. 3). All these authors found that Sagitta setosa overwintered mainly as stage I. Khan (1970), however, found stage II as dominant in winter at the Irish Sea. Resland (1983), working in the Kattegat, recorded very few stage I animals in late autumn-winter, and his results showed that the population at that time mainly consisted of animals at stage II. The different results obtained by Resland (1983) and Khan (1970) on one hand, and those of this study and of other authors (e.g. Pierce, 1941) on the other hand, could possibly be explained by discrepancies from author to author concerning the determination of the gonadic stages of the animals. This is supported by the fact that Resland (1983) found stage II smaller than 5 mm length. The minimum length for stage II found in this work was 5.80 mm, in September 1986. Minimum size for this stage recorded by Russell (1932 b) and Pierce (1941) was about 7 mm. There are also controversial findings concerning to the number of broods produced yearly by this species. Russell (1933) suggested five or six generations per year in the English channel, but his results were analyzed by Jakobsen (1971), who pointed out that Russell's results were difficult to interpret due to possible import from other Sagitta setosa populations. Pierce (1941) mentioned two possible breeding periods in the outer Mersey channels, one starting in April, which would extend until June, the second spawning in August. However, during the period from April through August, he found only small numbers of stage I (April), and these animals had already a minimum size of about 8 mm (his Fig. 2). In his results, no stage I was recorded from May through August, thus although he recorded gonadic stage III animals in April, spawning actually had no occurred until September, when small stage I were found. In the Kattegat, Resland (1983) also recorded Sagitta setosa as stage III from April-May, but no evidence of spawning was found. S. setosa is an allochthonous species in that area, and the animals are transported to that area as a mixture of adults and juveniles. Resland (1983) defined spawning as the presence of small stage I animals with less than 3 mm length. He found a life span to be one year in that area and pointed out that: "the appearence of stage III individuals is not an appropiate definition of breeding period since such individuals were also found at a time (April-May) when no breeding is evident". Khan and Williamson (1970), working in the Irish Sea, found low abundances for this species in April-May at all stations, and high abundance in August-September, mainly at stations 5, 6 and 7 (their Fig. 2). They pointed out: "Sagitta setosa has a prolonged breeding period with one peak in spring and another in late summer-autumn". Khan (1970) found animals less than 5 mm in March and April (his Fig. 3), and 2 mm specimens in August. However, this size animals were not found in spring neither by Russell (1932 b) in the Plymouth area nor by Pierce (1941) in the Irish Sea. Several authors (e. g. Williamson, 1956a) have acknowledged the difficulty of identifying Sagitta specimens of less than about 6 mm. This is important because Sagitta setosa and S. elegans overlap in their distribution at the sampled area. S. elegans spawning, however, is well stablished to occur mainly in spring not only for the Irish Sea but also for other regions (Russell, 1932a; Pierce, 1941; Jakobsen, 1971; Zo, 1973; O' Brien, 1976; King, 1979; Tande, 1983; Resland, 1983; Álvarez-Cadena, in press). Determining the number of broods for Sagitta setosa in this work is difficult because the species disappeared from the sampling area in spring-summer. However, if we combine the results obtained by Pierce (1941)with those obtained presently, it is probable that S. setosa has only one brood annually. This would imply the acceptance of the following assumptions, which are nonetheless not difficult to believe 1) As suggested by Resland (1983), the presence of stage IIIdoes not imply breeding. 2) The small numbers of stage I animals larger than 7 mm length recorded in April by Pierce (1941) are not the result of breeding at that time. The already large minimum size of the animals suggests instead that they are the progeny from the spawners of the preceding year. 3) The conditions (e.g. temperature) in Liverpool Bay and neighbouring sea areas are similar to those found in the sampled area. Consequently, the population found near the Isle of Man, which spreads from about June until December from the west coast of England into this area (see Williamson, 1952, 1956; Khan and Williamson, 1970), undergoes similar changes. Expanding on assumption 2, it is worth noticing that spawners in stage III can be found as late as November. Sagitta elegans hatches at about 1.2 mm length (Kotori, 1975), and although the hatching size of Sagitta setosa has not been reported, this species is smaller than the former and a similar or smaller hatching size would be expected. In the present work, stage I S. setosa animals were observed to increase in length by 1.38 mm between November 1986 and January 1987. No figures are available for February-April, but differences in temperature, between these months were less than 1ºC, and a similar size increment may be assumed. Thus, a specimen hatched in November would reach a length of ca. 8 mm in April, which would provide an explanation of the specimens found by Pierce (1941) at that time. If the three assumptions presented above are accepted, then the following theoretical picture of the life cycle of Sagitta setosa in the Irish Sea can be constructed. In the Irish Sea, Sagitta setosa breeds from about July-August to November; all the three stages are present throughout this period, stage I having a minimum length of less than 5 mm. The species overwinters from December to February mainly as stage I, with maturation to stage II of part of the population mainly in the latter month. No stage III animals are present at this time of the year. In April-May stage II becomes more abundant and the mean size increases. Animals at this stage are dominant at that time and part of the population motures to stage III. This maturation to stage III extends until June-July. However, no stage I would be present in spring or early summer or, if recorded, the relatively large minimum length of the specimens would identify them as originated from the brood of the preceding year. This situation, with no stage I specimens of less than 5 mm, would extend until June-July. In July-August, breeding starts again, and the life cycle is completed. Although this scheme seems reasonable, a more consistent sampling throughout the whole Irish Sea, not just near talle Isle of Man waters, is requierd to give definite results. AgradecimientosThis work was carried out at the Marine Biological Station Port Erin Isle of Man, Great Britain. I wish to thank my supervisor Dr. D. I. Williamson for his advice and encouragement. Dr. B.I. van Tussenbroek patiently and kindly revised, criticized and made suggestions for improving the manuscript. Dr. Lourdes Segura-Puertas revised the final version. This is a part of a Ph. D. dissertation made possible through a grant provided by The British Council and the Consejo Nacional de Ciencia y Tecnología (CONACyT), México (Grant No. 49271). LITERATURAÁLVAREZ-CADENA, J. N. (in press). Life cycle, gonadic stages and size frequency distribution of the chaetognath Sagitta elegans Verrill in the northeastern Irish Sea. Est. Coast. Shelf Sci. ALVARIÑO, A., Oceanog. Mar. Biol. An Ann. Rev., Chaetognaths. In: H. Barnes (Ed.). 1965 115-195 3 BAJKOV, A. D. How to estimate the daily food consumption of fish under natural conditions. Trans. Amer Fish Soc., 1935 288-289 65 DODGE, J. D., Marine Dinofiagellates of the British Isles. Her Majesty's Stationary Office. London. 1982 303 FEIGENBAUM, D., and R. C. MARIS., Oceanog. Mar. Biol. An Ann. Rev., Feeding in the Chaetognatha. 1984 343-392 22 GIESBRECHT, W., Atlas 54. Pelagischen Copepoden des Golfes von Neapel Verlag von R. Friedländer & Sohn. Berlin. 1892 831 GRAZIANO, C., On the ecology of Tintinnids (Ciliophopora: Oligotrichida) in the north Irish Sea. Est. Coast. Shelf Sci., 1989 233-245 29 JAKOBSEN, T., On the biology of Sagitta elegans Verrill and Sagitta setosa J. Müller in inner Oslofjord. Norw. J. Zool., 1971 201-225 19 KHAN, M. A., Thesis University of Liverpool. The distribution of planktonic species and hydrographical factors in the Eastern Irish Sea. M. Sc. 1970 43 KHAN, M. A., and D. I. WILLIAMSON, Seasonal changes in the distribution of Chaetognatha and other plankton in the eastern Irish Sea. J. Exp. Mar. Bio. Ecol., 1970 285-303 5 KING, R. K., The life history and vertical distribution of the chaetognath Sagitta elegans in Dabbob Bay, Washington. J. Plankton Res. 1979 153-167 1 2 KOTORI, M., Morphology of Sagitta elegans (Chaetognatha) in early larval stages. J. Oceanogr. Soc. Japan, 1975 139-144 31 LEE, J. W., Thesis. Observations on the Vertical Distribution of Plankton at Different Stations in the Irish Sea. Ph. D. University of Liverpool. 1971 228 MIRONOV, G. N., The food of plankton predators. II. Food of Sagitta. Trudy Sebastopol Biol. Sta., 1960 78-88 13 O'BRIEN, F. I., The life cycle of Sagitta elegans Verrill and Sagitta setosa J. Müller in Galway Bay, West coast of Ireland. J. Mar. Biol. Ass. U. K., 1976 191-196 56 RESLAND, V., Abundance, breeding and temporal size distribution of the chaetognath Sagitta setosa in the Kattegat. J. Plankton Res. 1983 425-439 5 _____, Feeding of the chaetognaths Sagitta elegans and S. setosa at different seasons in Gulmarsfjorden, Sweden. Mar. Ecol. Prog. Ser., 1987 69-79 39 PEARRE, S. Jr., Invest. Pesq., Ecological studies of three west-Mediterranean chaetognaths. 1974 325-369 38 PIERCE, E. L., The ocurrence and breeding of Sagitta elegans Verrill and Sagitta setosa J. Müller in parts of the Irish Sea. J. Mar. Biol. Ass. U. K., 1941 113-124 25 REEVE, M. R., Marine Food Chains. The biology of chaetognaths. I. Quantitative aspects of growth and egg production in Sagitta hispida. In: J. H. Steele (Ed.). Oliver and Boyd. Edinburg, 1970 168-189 ROSE, M., Faune de France. Conepodes Pélagiques. 26 Office Central de Faunistique. Paris. 1933 374 RUSSELL, F. R., On the biology of Sagitta. I. The breeding and growth of Sagitta elegans Verrill in the Plymouth area, 1930-1931. J. Mar. Biol. Ass. U. K., 1932a 131-146 18 _____, On the biology of Sagitta. II. The breeding and growth of Sagitta setosa Müller in the Plymouth, area, 1931-1932. J. Mar. Biol. Ass. U. K., 1932b 146-161 18 _____, On the biology of Sagitta. III. A further observation on the growth and breeding of Sagitta setosa in the Plymouth area, 1930-1931. J. Mar. Biol. Ass. U. K., 1933 555-558 18 2 _____, On the values of certain plankton animals as indicators of water movements in the English Channel and North Sea. J. Mar. Biol. Ass. U.K, 1935 309-332 20 _____, Hydrographical and biological conditions in the North Sea as indicated by plankton organisms. J. Cons. Perm. In Expl. Mer., 1939 171-192 14 SHELBOURNE, J. E. A predatory-prey size relationship for plaice larvae feeding on Oikopleura. J. Mar. Biol. Ass. U. K., 1962 243-252 42 SLINN, D. J., and J. F. EASTHAM Ann Biol, Routine hydrographic observations in the Irish Sea off Port Erin Isle of Man, during 1972-1981 inclusive. 1984 42-43 38 SULLIVAN, B. K, C. B. MILLER, W. T. PETERSON, and A. H. SOELDNER A scanning electron microscope study of the mandibular morphology of boreal copepods. Mar. Biol., 1975 175-182 30 TANDE, K. S., Ecological investigations of the zooplankton community of Balfsjorden, northerm Norway: population structure and breeding biology of the chaetognath Sagitta elegans Verril. J. Exp. Mar. Biol. Ecol., 1983 13-24 68 TREGOUBOFF, G., and M. ROSE. Manuel de Planctonologie Méditerranénne. Tome I and II. Centre Nacional de la Réacherche Scientifique. Paris. 1957 587p, 207 figs. WILLIAMSON, D. I. Distribution of plankton in the Irish Sea in 1949 and 1950. Proc. Dan Lpool. Biol. Soc. 1952 1-46 58 _____, Bull. Mar. Ecol., The plankton in the Irish Sea, 1951 and 1952. 1956 87-114 4 ZO, Z Limnol. Oceanogr., Breeding and growth of the chaetognath Sagitta elegans in Bedford Basin. 1973 750-756 18
|

