|
HEAVY METALS
DISTRIBUTION IN GEOCHEMICAL FRACTIONS OF SURFACE SEDIMENTS FROM THE LOWER GULF
OF CALIFORNIA
Recibido el 17 de mayo de 1990 y aceptado para su
publicación el 12 de julio de 1990.
Federico Páez-Osuna
José I. Osuna-López
Instituto de
Ciencias del Mar y Limnología, UNAM, Estación Mazatlán,
Apartado Postal 811, Mazatlán, Sinaloa, 82000, México.
Contribución No. 686 del Instituto de Ciencias del Mar y
Limnología, UNAM.
En este estudio se
realizaron extracciones químicas secuenciales en los sedimentos
superficiales (0-5 cm) del bajo Golfo de California con objeto de conocer la
participación geoquímica de los metales pesados. Fueron separadas
cuatro fracciones geoquímicas en los sedimentos y las concentraciones de
metales nos fueron determinadas en cada una de las 19 muestras por
técnicas de absorción atómica. La mayoría de los
metales estuvieron asociados principalmente a la fracción
litogénica, los cuales son los componentes más abundantes en las
depresiones de las cuencas. En ambos rasgos morfológicos se
observó que Fe, Cu y Co tienen las concentraciones más elevadas
en la fracción reducible que en la oxidable, contrario al Ni, Cr, Pb y
Mn. En la mayoría de las muestras el Cd mostró proporciones altas
(hasta de 100%) en el extracto de peróxido.
In this study
sequential chemical extractions were carrided out to determine the geochemical
partition of heavy metals in surface sediments (0-5 cm) collected from the
lower Gulf of California. Four geochemical fractions of the sediments were
separated and the concentrations of heavy metals (Cd, Cu, Co, Cr, Fe, Mn, Ni
and Pb) were determined in each of the 19 samples by atomic absorption
techniques. Most of the metals were associated principally to the lithogenic
fraction and are major components in the continental shelf and minor components
in the basins depressions. In both morphologic features it was observed that
Fe, Cu and Co have higher concentrations, in the reducible than in the
oxidizable fraction, contrary to Ni, Cr, Pb and Mn. Cd in most of samples
showed high proportions (ca 100%) in the peroxide extract.
Sediments are complex mixtures of a number of solid phases that may include organic matter, clays, carbonates, silica, metal oxides and other minerals. Consequently the heavy metals can be associated with various operationally defined geochemical phases (Tessier and Campbell, 1987): (1) adsorbed at particle surfaces, e.g., clays, humic acids, metal oxyhydroxides; (2) bound up with organic matter in either living or detrital form, and sulphide bound (e.g. amorphous sulphides formed in situ or more crystalline forms); (3) carbonate-bound (e.g. discrete carbonate minerals co-precipitated with major carbonate phases); (4) occluded in iron and/or manganese oxyhydroxides (e.g. discrete nodules, cement between particles, coatings on particles); (5) residual or lithogenic matrix-bound (e.g. bound in lattice positions in aluminosilicates resistant oxides or sulphides) in order to estimate the distribution of metals among specific geochemical phases, several analytic techniques has been developed (Goldberg and Arrhenius 1958; Chester and Hughes, 1967; Bruland et al, 1974; Gupta and Chen, 1975; Gibbs, 1977; Tessier et al, 1979; Calmano and Forstner, 1983) The purpose of this work was to examine surface sediments of the lower Gulf of California, to differentiate them into four geochemical fractions. The distribution of eight metals Cd, Co, Cr, Cu, Fe, Mn, Ni and Pb, was estimated in each of these. METHODS AND STUDY AREAThe Gulf of California is located between the Baja California Península and mainland México. In addition to supporting a substantial fishery, the Gulf has long been recognized as an important area for oceanographic research because of its significance as a marginal sea (Badan-Dangon et al, 1985). The Gulf of California is a long (1100 km) and narrow sea divided into two major physiographic regions, the upper and the lower Gulf. The lower Gulf considered here, south of the midriff islands around 29ºN, has a series of basins which progressively deepen to oceanic depth towards the mouth, and is in open comunication with the Pacific (Baumgartner et al, 1985). An ubiquitous feature of the central and southern Gulf is the presence of a band of hemipelagic, laminated, diatimaceous sediments. The accumulation and preservation of laminated sediment in this portion of the Gulf is maintained by an oxygen minimum layer within the water column between 500 and 1000 m depth and by the annual cycle of regional climate which controls the composition of sediment arriving to the sea floor (Soutar et al, 1981). Sediment distribution in the Gulf has been described in detail Byrne and Emery, 1960; Van Andel, 1964; Calvert, 1964, 1966). Briefly, the sediments in the lower Gulf have been identified to consist mainly of silty clay, while the sediments adjacent to the coast are composed predominantly of sands with variable proportions of shell material, silts, and clays. Reconnaissance geochemical prospecting techniques were employed in the lower Gulf. The delineation of several heavy metal anomalies (Fe, Mn, Cu, Ni, and Zn) in the sediments was found (Niemitz, 1977), these anomalies solely correlative with inferred tectonic elements remain as permissive evidence of hydrothermal input to the sediments. (Fig. 1). METHODSSediments samples from 19 stations were collected from the lower Gulf of California (Fig. 1) using a gravity corer (Páez Osuna et al, 1986). As soon as a core was on deck, it was cut into 5 cm sections; only the top (0.5 cm) was selected and analyzed in this study. Sediment samples were placed in plastic bags, sealed in argon atmosphere and frozen for transport to Mazatlán. In the laboratory the samples were dried in an oyen at 90ºC, homogenized and reduced to a fine powder in a mortar. TOTAL LEACHABLE HEAVY METAL DETERMINATIONSSamples were leached at about 130ºC with a 3:1 mixture of HN03 and HCI (Breder, 1982) in a teflon decomposition manifold system (Stoeppler and Backhaus, 1978). The residues after digestion were centrifuged, and the diluted solutions were analyzed by flame AAS (Shimadzu AA-630) using the manufacturer's conditions. The standard errors for this method were: Cu, 5%; Cd, 8%; Co, 5%; Cr, 10%; Fe, 5%; Mn, 8%; Ni, 4%; Pb, 9%; and Zn, 6%. An IAEA sediment standard SD-N-1/2 (IAEA, 1985), treated in the same ways as the samples, was used to ensure the accuracy of the analysis. SELECTIVE EXTRACIONAfter an evaluation of the literature we chose the method described below. The procedure selected is the proposed by Meguellati et al, (1983), which is an adaptation of sequential method of Tessier et al, (1979). The main difference with the Tessier procedure is the extraction of the organic phases after the exchangeable phases (Table 1). This allows the destruction of organic matter which has entrapped the mineral materials and thus provides better extraction for the following fractions. (Table 1). RESULTS AND DISCUSSIONThe distributions of the metals in the various fractions are presented in Tables 2-9; for the different morphologic features of the central and southem Gulf of California: three core samples (0-5 cm) B1 (depth z=240 m), B7 (z=45 m) and B37 (z=25 m) were employed for the continental shelf. B22 (Z=1350 M), B25 (Z=2450 M) from the sills among Carmen and Guaymas, Farallón and Pescadero basins respectively, and B3 (Z=2500 m), B4 (Z=1210 m), B9 (Z=1240 m), B12 (Z=670 m), B17 (Z=790 m), B18(Z=380 m), B19 (Z=1500m), and B20 (Z=1490) stations from the slope and edge of basins were averaged and denominated "slope" in Table 2-9; for depressions of the basins the top (0-5 cm), of the cores B14 (Z=2020 m) from Guaymas basin were used, BS (Z=2250 m), B6 (Z=2220 m), and B24 (Z=3250 m ) from Farallón basin, B26 (Z=3230 m) from Pescadero basin and B29 (Z=3180 m) from Mazatlán basin. The results are discussed individually for each metal below. 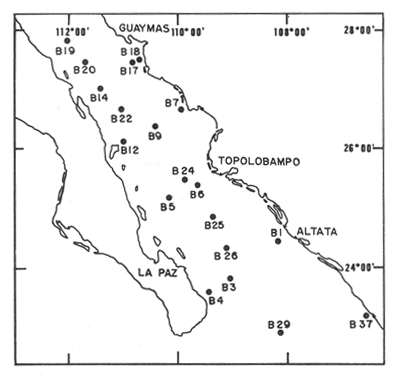 Figura 1. Study arca showing the location of sampling sites. CADMIUMIn contrast to other studies (Gupta and Chen, 1975; Helsinger and Friedman, 1982; Lion et al, 1982; Rapin et al, 1983; Abaychi and Douabul, 1986; Rosental et al, 1986) where most Cd was found in association with the carbonates and reducible fractions in this study, about an average of 61% of the total Cd in most samples was found in oxidizable fraction (Table 2) These results may be due to distinct characteristics of sediments, or to a different sequential extraction procedure used for the partitioning of metals. Organic sedimentation, and the presence of an oxygen minimum layer for example is a ubiquitous feature of the Gulf of California in opposition to False Bay, SW Africa (Rosental, et al, 1986) or NW Arabian Gulf (Abaychi and Douabul, 1986). Although hydroxylamine in 25% acetic acid was originally designed to be used on oxidized sediments to extract metals associated with hydrous oxide phases of manganese and iron (Chester and Hughes, 1967), it will also extract acetic acid soluble forms of the metals from both oxidized and reduced sediments (Gendron et al, 1986). Thus in reduced sediments, this Cd extracted may be erroneusly distinguished as associated to reducible phases. The predominance of Cd in the organic/sulphide fraction can be explained based on the fact that several studies of cadmium in the deep sea have shown that the biogeochemistry of cadmium is strongly dominated by the organic matter cycle. Martin et al, (1976), Bruland (1980) and Knauer and Martin (1981) have proposed that cadmium in the marine environment is fixed by phytoplankton in the surface water and transported toward the floor with the remains of these organisms. When organic detritus is decomposed, cadmium is liberated with other mineralization products. Our data is consistent with an involvement of cadmium with the organic fraction, due to the differences in preservation conditions among the continental shelf, slope and depressions of basins from California Gulf, which are reflected in their distinct concentrations. (Figure 2). 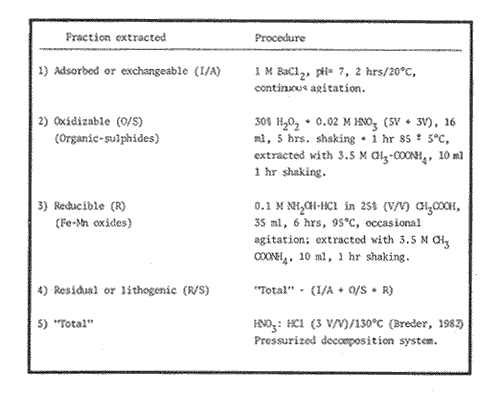 TABLE 1 SEQUENTIAL EXTRACTION PROCEDURE ADAPTER AFTER TESSIER et al (1979) AND MEGUALLATI et al 1983. CHROMIUMCr was contained very largely in the lithogenic fraction (Table 3); this fraction has an average of 74% of the total chromium. These values are relatively high in comparison to False Bay (Rosental et al, 1986), or Villefranche Bay (Rapin et al, 1983), but comparable to the proportions given for the Arabian Gulf (Abaychi and Douabul, 1986). Some researchers (Turekian 1967; Chester and Messiha-Hanna, 1970) have suggested that chromium and other metals are elements of detrital origin in the pelagic sediments, so that the results found in the hemipelagic sediments of the Gulf of California are not surprising. Non lithogenic chromium is associated mainly to the oxidizable fraction on the continental shelf and the depressions of basins, though on the slopes the non-lithogenic fraction of this element is deposited principally in the reducible fraction (Figure 2). COPPERThe non-lithogenic copper (Table 4) from the continental shelf is associated principally to iron and or manganese oxyhydroxides (18.2%) while in the slope and the basin's depressions from the lower Gulf of California, the metal is found predominantly bound up with organic matter and sulphides (19.6 and 23.8% respectively). This last observation has been explained by Rosental et al, (1986), pointing out that numerous workers who have found elevated concentrations of Cu in sediments have also suggested that the major form of Cu were Cu-organic complexes (Tessier et al, 1979; Rapin et al, 1983). In table 10, copper proportions are shown as percentages of total metal (Cu, Co and Ni) in the oxidizable, reducible and residual fractions of Saanich Inlet (Presley et al, 1972), California basins, (Bruland et al, 1974) and California Gulf sediments. The singular difference between the Gulf of the other regions, is the relatively elevated proportion of reducible - Cu (18-22%) in the Gulf, even though during their extrations carbonates and sulphide copper fractions were also probably included. (Tables 4 y 5). 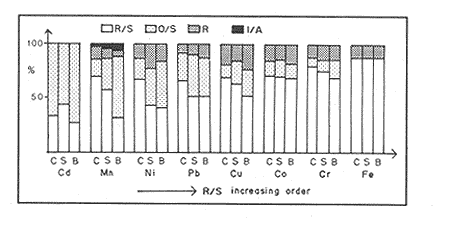 Figure 2. Average percentages of the different fractions of elements extracted from surface collected in the lower Gulf of California. Morphologic features: C, continental shelf S, A basin depressions. Fractions: II/A, exchangeable'; OIS, 'organic matter plus sulpjides'; R, \Fe and Mn oxides'; R/S, 'residual'. 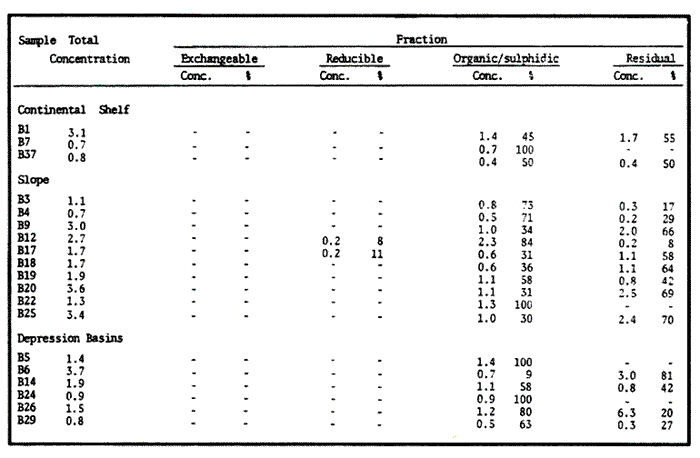 TABLE 2 DISTRIBUTION OF Cd IN SEDIMENT FRACTIONS (CONC. IN PPM) 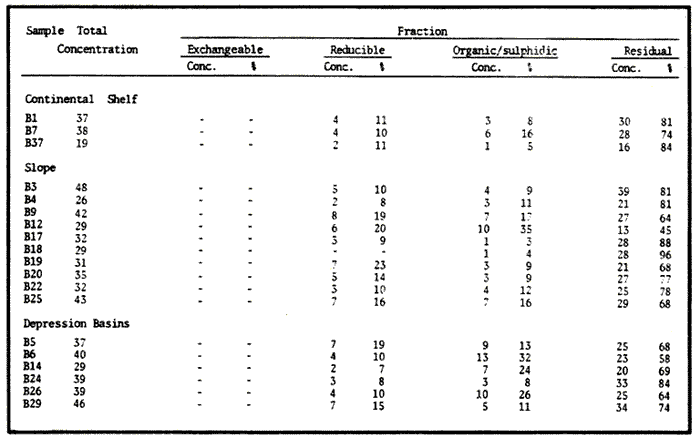 TABLE 3 DISTRIBUTION OF Cr IN SEDIMENT FRACTIONS (CONC. IN PPM) COBALTApproximately between 47 and 85% of the Co found, was associated with the residual fraction (Table 5). In the other fractions, and important proportion is bound to hydrous Mn/Fe oxides on the surface sediments of continental shelf (mean of 17.3%) and the basin's depressions (mean of 17.5%), while on the slope, this element is deposited in the non-lithogenic fraction predominantly bound with organic matter and sulphides (Figure 2). In comparison to other regions the sediments of the Gulf of California have major concentrations of Co in the reducible and oxidizable fractions, than the sediments from Saanich Inlet or the California basins (Table 10). The slope, where a strong oxygen minimum zone intercepts the sediment water interface, has the lowest concentrations of hydroxylamine/acetic acid extractable cobalt (Figure 2), which is consistent with the behavior of this element. In contrast to cadmium, cobalt is more lithogenic and has a strong affinity for manganese and iron oxides, which has been proposed by various investigators (Goldberg, 1954; Heggie and Lewis, 1983; Gendron et al, 1986). NICKELAn average of about 66% in the continental shelf, 42% in slope and 39% in basin depressions of the total Ni was found in the residual fraction (Table 6). The rest is associated principally to the oxidizable phase (mean of 20 to 46%), though a significant proportion (mean of 10 to 20%) is accumulated in the fraction removed with hydroxylamine hydrochloride in acetic acid. Thus the Ni potentially available in the Gulf of California False Bay (Rosental et al, 1986), Mobil Bay (Branon et al, 1977) or the depression of Saanich Inlet (Presley et al, 1972) is found predominantly associated to the oxidizable fraction (Table 10). LEADWith a range of 10-76% of organic sulphides (and possibly carbonates) bound of total Pb is deposited as the most important non-lithogenic fraction in the three morphologic features of the lower Gulf of California (Table 7). This observation contrast with other studies (Rapin et al, 1983; Abaychi and Douabul, 1986; Rosental et al, 1968) where non-lithogenic Pb predominante as part of Fe/Mn oxides and hydroxides However it is difficult to guarantee such affirmation since by the peroxide extractíon some metals bound to amorphous forms of Fe/Mn oxides and hydroxides could have been leached. IRONAn average of 87.7%, 88.5% and 88.2% of Fe was found in the lithogenic fraction of the continental shelf, slope: and depressions sediments (Table 8). The remainder is deposited principally in the reducible fraction (6.0-18.2%). A small percentage (0.2-4.2%) is accumulated as part of the organic sulphide fraction. The reducible level (mean of 10.3%) found in this study is comparable to the values reported in other studies (Chester and Messiha-Hanna, 1970; Fóster and Hunt, 1975; Deurer et al, 1978), although it is minor in comparison to regions as False Bay (Rosental et al, 1986) or Villefranche Bay (Rapin et al, 1983). (Tables 8 y 9). MANGANESEThe speciation of manganese differs from that of the preceding metals, in that the exchangeable fraction contributes with appreciable proportion (Ca 13%) to the metal concentration. The Mn in the lithogenic fraction was very variable (Table 9). This highest percentage in this phase was found in the continental shelf samples (X=69%), and the lowest (X=31%) in the basin depresions. The rest is associated predominantly to the oxidizable fraction, with 58, 20 and 17% in the surface (0-5 cm) sediments from the depressions, slope and continental shelf. Slope sediments of the lower Gulf are in contact with oxygen depleted waters between 400 to 800 m (Schrader and Baumgartner, 1983). Thus the elevated percentage of Mn found in the oxidizable fraction is not surprising in these sediments, but this observation is rather unusual in the basins depressions since it has a positive redox of 114 to 360 mv in the top (0-5 cm) sections of cores (Páez - Osuna, 1988), which is favourable for the preservation (or formation) of Fe-Mn oxides. However some investigations (Tipping, 1981; Laxen, 1985) have demostrated and evident interaction between the humic subtances and oxides. Consequently, if the Fe-Mn oxidel are acting as a substrate for a thin adsorbed organic film it is possible that some metals adsorbed on amorphous Fe-Mn are released with the peroxide attack. (Table 10). 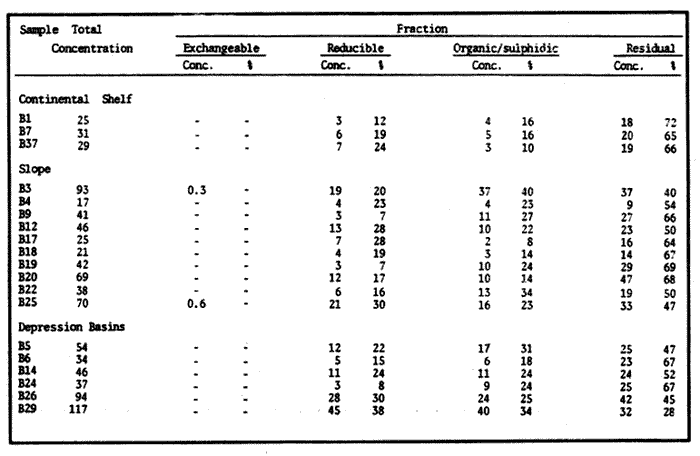 TABLE 4 DISTRIBUTION OF Cu IN SEDIMENT FRACTIONS (CONC. IN PPM) 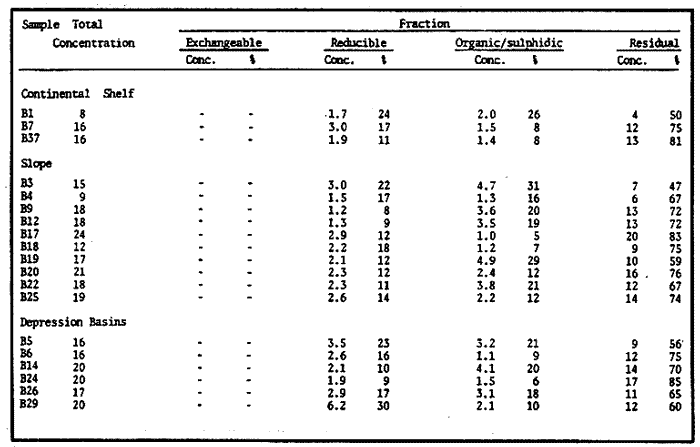 TABLE 5 DISTRIBUTION OF Co IN SEDIMENT FRACTIONS (CONC. IN PPM) 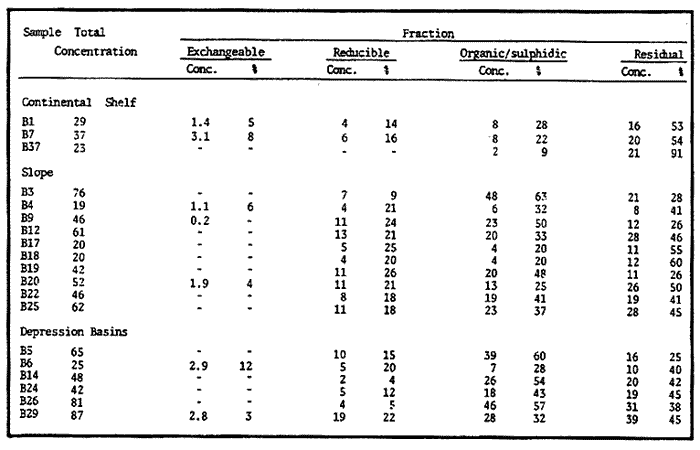 TABLE 6 DISTRIBUTION OF Ni IN SEDIMENT FRACTIONS (CONC. IN PPM) 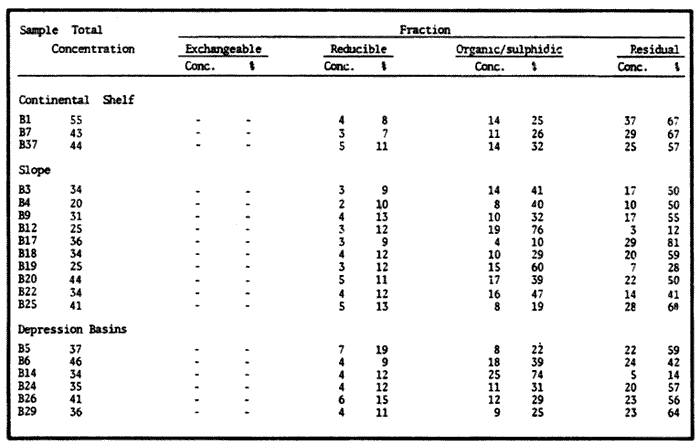 TABLE 7 DISTRIBUTION OF Pb IN SEDIMENT FRACTIONS (CONC. IN PPM) 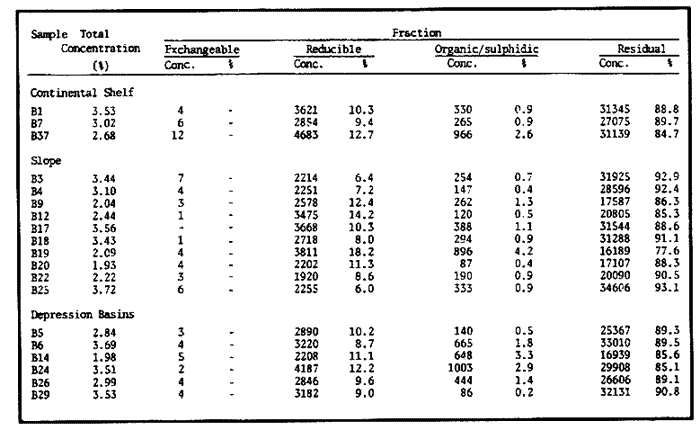 TABLE 8 DISTRIBUTION OF Fe IN SEDIMENT FRACTIONS (CONC. IN PPM) 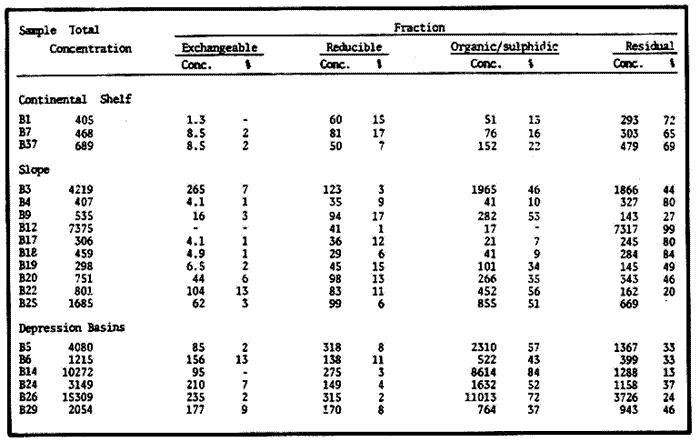 TABLE 9 DISTRIBUTION OF Mn IN SEDIMENT FRACTIONS (CONC. IN PPM) 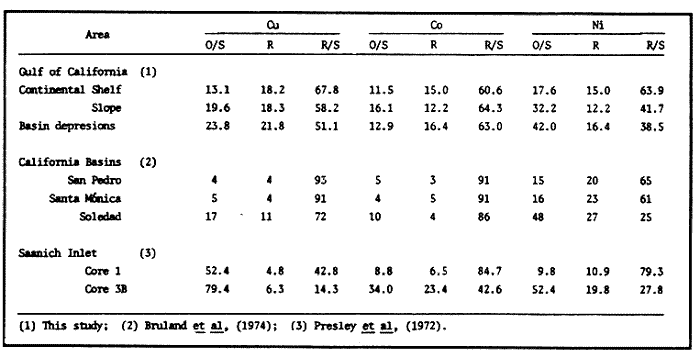 TABLE 10 Cu, Co AND Ni IN THE OXIDIZABLE (O/S), REDUCIBLE (R) AND RESIDUAL (R/S) FRACTION AS PERCENTAGE OF THE SUM, IN ORGANIC CARBON-RICH HEMIPELAGIC SEDIMENTS ConclusionesIn lower Gulf of California sediments, the heavy metal partitioning pattern of the elements studied show certain similarities, but also significant differences. In general, highest levels of the metals investigated (with the exception of Cd, Mn and Ni) were found in the residual fractions of the sediments. The lithogenic content decreased in the following order: Fe>Cr>Co> Cu>Pb>Ni>Mn>Cd (Figure 2). Fe, Pb, Co and Cr responded similary to each other in the hydroxylamine hydrochioride-acetic extraction. A relatively small percentage (Figure 2, 9-18%) of the total concentration in the three morphologic features of the lower Gulf of California sediments was removed. The metals in this fraction could be associated with carbonate or hydrate oxide phases in the sediments. Most of the Cd was removed with the peroxide attack. It therefore seems that Cd occurs as sulphides or organic forms in the surface sediments of the lower gulf. No significant differences in the metal partitioning pattems among the continental shelf, slope and basin sediments could be observed (Figure 2). Only Mn displayed a certain amount of variation, which is probably due to different redox conditions in each part of the gulf, moreover of the hydrothermal contribution from the southem trough of Guaymas Basin (Campbell 1985). The sample B14 of the hydrothermal field, showed anomalous proportions in the organic/sulphidic fraction for Mn (84%), Pb (74%) and Fe (3.3%). This reflects in the material the well known presence of amorphous Mn, galena, marcasite and pyrrhotite minerals (Peter et al, 1987; Ortega - Osorio and Páez Osuna, 1989). AgradecimientosThe institutional and financial support for the present study was provided by the Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México (ICMyL, UNAM) and the UNAM-CONACyT proyect PCCBBNA - 021 995. The authors wish to thank Jeanette Wolf Villegas for reviewing the English manuscript and to Dr. E.D. Goldberg for his critical review of the manuscript. Thanks too to Margarita Cordero for secretarial assistance. LITERATURAABAYCHI, J.K. and A.A.Z. DOUABULGulf. Mar. Poll. Bull. Trace element Geochemical associations in the Arabian 1986. 353 - 356.17. BADAN-DANGON, A. C.J. KOBLINSKY. and T. BAUMGARTNER Oceanologica Acta Spring and Summer in the Gulf of California: Observadons of surface thermal patterns. 1985.13 - 22. 8 BAUMGARTNER T. V. FERREIRA-BARTRINA, H. SCHRADER and A. SOUTAR A 20 year varve record of Siliceous phytoplankton variability in the Central Gulf of California.Mar. Geol.1985. 113 - 129. 64 BRANNON J.M. J.R. ROSS, R.M. ENGLER y I. SMITH Ann. Arbor. Science. An Arbor. The distribution of heavy. metals in sediment fractions from Mobile Bay, Alabama In: Yen, T.F. (Ed). 1977. 125 - 149 p. BREDER, R. Fresenius Z. Anal. Chem. Optimization studies for realiable trace metal analysis in sediments by atomic absorption spectrometric methods. 1982. 395 - 402. 313: BRULAND, K.W. K. BERTINE, M. KOIDE and E.D. GOLDBERG History of metal pollution in southerri California Coastal zone.Environ Sci. Technol.1974. 425 - 432. 8: BRULAND KW. Oceanic distributions of Cadmium, Zinc, Nickel and Copper in the North Pacific. Earth Planet. Sci. Lett. 1980. 176-198. 47: BYRNE, J.V. and L.P. EMERY Geol. Soc. Am. Bull.Sediments of the Gulf of California. 1960. 983 - 1010. 71: CALMANO, W. and U. FORSTNER Chemical extraction of heavy metals in polluted river sediments in Central Europe.Sci. total Environ.1983. 77 - 90. 28: CALVERT, S.E. Ph. Doctor thesis The diatomaceous sediments of the Gulf of California. University of California San Diego1964.235p. CALVERT, S.E Geol. Soc. Am. Bull. Accumulation of diatomaceous silica in the sediments of the Gulf of California.1966. 569 -596. 77 CAMPBELL, A.G. Ph. Doctor thesis. Geochemistry of hydrothermal clouds in the Guaymas Basin, Gulf of California. University of California San Diego1985. 262 p. CHESTER W and M.J. HUGHESChem. Geol. A chemical technique for the separation of ferromanganese minerals, carbonate minerals and adsorbed trace elements from pelagic sediments1967.249-262. 2: CHESTER R. and R.G. MESSIHA-HANNA Acta Trace element partition patterna in North Atlantic deep sea sediments. Geochim Cosmochim. 1970.1121 - 1128. 34 DEURER R.U. FORSTNER and G. SCHMOLL Acta Selective chemical extraction of Carbonate associated metals from recent lacustrine sedimenta. Geochim Cosmochim. 1978. 425 - 427. 42 FOSTER. P. and D.T.E. HUNT Mar. Geol Geochemistry of surface sediments in an acid stream estuary1975. 13 - 21. 18: GENDRON A.N. SILVERBERG, B. SUNDBY and J. LEBEL Bull. Am. Geol. Soc. Early diagenesis of cadmium and cobalt in sediments of the Laurentian Trough. Geochim Closmochim. Acta SO: 741 - 747. GIBBS R.J. 1978. Transport phases of transition metals in the Amazon and Yukon rivers.1986. 829 - 843. 88 GOLDBERG E.D. J. Geol. Marine geochemistry chemical scavengers of the Sea.1954. 249 - 266. 62: GOLDBERG ED. and G.O. ARREHENIUS ActaChemistry of Pacific pelagic sediments. Geochim Cosmochim. 1958.153 - 212.13 GUPTA S.K. and K.Y. CHENEnviron. Lett Partitioning of trace metals in selective chemical fractions of near-shore sediments.1975. 129 -158. 10: HEGGIE D. and T. LEWIS Nature Cobalt in pore waters of marine sediments 1983. 453 - 455.311 HELSINGER M.H. and G.M. FRIEDMAN O.M. Distribution and incorporation of trace elements in the bottom sediments of the Hudson River and its tributaries.Northeastern Enviaran Sci. 1982. 33 - 47. 1: INTERNATIONAL ATOMIC ENERGY AGENCY Intercon of Trace element measurements in marine sediment samples SD-N-1/2 Report Mónaco1985. 74 p. No. 24 KNAUER G.A. and J.H. MARTIN J. Marine Res. Phosphorus-Cadmium cycling in Northeast Pacific waters.1981. 65 - 76. 39: LAXEN D.P.H. Trace metal adsorption/coprecipitation os hydrous ferric oxide un realistic conditions. Water Res.1985.1229-1236.19: LION L.W. R.V. HARVER and J.O. LECKIE Mechanisms for trace metal enrichment at the surface microlayer in an estuarine salt marwh. Mar. Chem. 1982. 236 - 244. 11: MEGALLITI N. D. ROBBE P. MARCHANDISE and M. ASTRUC A new chemical extraction procedure in the fractionation of heavy metals in sediments interpretation. In Proceedings of the internacional conference. Heavy metals in the environment September 1983 Heidelberg Germany 1983. 4 p. MARTIN HJ. K.W. BRULAND and W.W. BROENKOW Marine pollution Transfer Lexington Books, Cadmium transport in the California Current. In: H.L Windom, and R.A. Duce (Eds). Toronto 1976. 159-184p. NIEMITZ J.W. Ph. Doctor Thesis Tectonics and geochemical exploration for metal deposits in the southern Gulf of California University of Southern California San Diego1977. 354 p. ORTEGA-OSORIO A. and F. PAEZ-OSUNA F. Composición geoquímica y Mineralógica de los dépositos hidrotermales de la Dorsal del Pacífico Mexicano (21ºN) y Cuenca de Guaymas. Geof. Int.28 1989. 737-762. 4: PAEZ-OSUNA F. J.I. OSUNA-LOPEZ L. D. MEE-MILLER and M.I. ABDULLAH. An. Inst. Cienc. del Mar y Limnol. Univ. Nal. Autón. México. Modificación a un nuclear ligero y económico para muestrear sedimentos sin disturbarlos: Nota Científica. 1986. 449 - 454.13: PAEZ-OSUNA F. Tesis Doctoral Geoquimica de los metales en los sedimentos del Mar de Cortés. UACPyP, CCH, UNAMMéxico1988. 392 p. PETER J.M. S.D. SCOTT and W.C. SHANKS Mineralogy and geochemistry of hydrothermal vent deposits in the southern trough of Guaymas basin, Gulf of California. NSF, Conference on the Mexican-American Guaymas Basin Expedition, 1985.MazatlánMéxico. 1987. 3 p.,(Abstract) PRESLEY BJ. Y. KOLODNY, A. NISSENBAUM and I.R. KAPLAN Acta Early diagenesis in a reducing fjord, Saanich Inlet, Brithish Columbia - 11. Trace element distribution in interstitial water and sediment. Geochim Cosmochim. 1972. 1073 - 1090. 36: RAPIN F. G.P. NEMBRINI, U. FOSTNER and J.I. GARCIA Env. Techn. Lett. Heavy metals in marine sediment phases determined by sequential chemical extraction and their interaction with interstitial water. 1983.387 - 396. 4 ROSENTAL R. G.A EAGLE and M.J. ORREN Trace metal distribution in different chemical fractions of Nearshore Marine Sediments. Est. Coast. shelf. Sci. 1986.303 - 324. 22: SCHRADER H. and T. BAUMGARTNER Coastal Upwelling its sediment record. Part B: Sedimentary Records of Ancient Coastal Upwelling. Decadal variation of upwelling in the Central Gulf of California. In: J. Theide and E. Suess (Eds) Plenum Press New York 1983. 247-276p. SOUTAR, A. S.P. JOHNSON and T. BAUMGARTNER The Monterrey Formation and related siliceous rocks of California, Search of modem depoisitional analogs to the Monterrey Formation In: Garrison, G.E. and R.G. Douglas (Eds). Soc. of Econ. Paleon. and Min.1981. 123- 147 p. STOEPPLER, M. and F. BACKHAUSFresenius Z. Anal. Chem. Pretreatment studies with Biological and Environmental Materials. 1978. 116 -120. 291 TESSIER, A. P.G.C. CAMPBELL and M. BISSON Anal. Chem. Sequential extraction procedure fro the speciation of particulate Trace Metals.1979.844-851. 51: TESSIER A. and P.G.C. CAMPBELLHydrobiologia Partitioning of trace metals in sediments: Relationships with bioavailiability.1987. 43 - 52. 149: TIPPING. E. Acta The adsorption of aquatic humic substances by iron oxides. Geochim Cosmochim. 1981. 191 - 199. 45: TUREKIAN, K.K. Progress in Oceanography Estimates of the average Pacific deep sea clay accumulation rate from material balance considerations.1967. 226 - 244. 4: Van Andel Tj. H. Marine Geology of the Gulf of California a Symposium. Recent marine sediments of Gulf of California. In: Van Andel Tj. H. and G.G. Shor (Eds). The American Association of Petroleum Geol. Mem. e.1964. 216 -310 p.
|

