|
POPULATIONS STUDY OF THE PENAEID SHRIMP OF TERMINOS LAGOON, CAMPECHE,
MEXICO
Trabajo recibido el 16 de marzo de 1990 y aceptado para su
publicación el 26 de octubre de 1990.
Adolfo Gracia G.
Luis A. Soto G.
Instituto de Ciencias del Mar y
Limnología, UNAM. México, D. F. Apartado postal 70-157.
México 04510, D. F. México. Contribución No. 689 del
Instituto de Ciencias del Mar y Limnología, UNAM.
Se analiza la distribución y
abundancia de las etapas estuarinas de los camarones peneidos Penaeus
duorarum, P. setiferus y P. aztecus La
inmigración de postlarvas planctónicas a la Laguna de
Términos se lleva a cabo durante todo el año, con máximos
en julio y septiembre. P. setíferus y P.
duorarum son las especies que ingresan principalmente a la laguna.
Los juveniles de las tres especies presentan un patrón de
distribución espacio-temporal distinto en el área estuarina. El
camarón blanco y el camarón café emplean en forma
temporalmente diferencial el habitat con sustratos suaves situado al suroeste
de la Laguna de Términos. El camarón rosado muestra una
segregación espacial con respecto a las dos especies anteriores, ya que
su distribución principal es en el área este, sobre sustratos
calcáreos. Se identifican dos períodos de alta y baja abundancia
en las etapas estuarinas de los camarones peneidos. La variabilidad en la
abundancia es mayor en las primeras etapas. En un ciclo anual se presentan dos
cohortes dominantes de P. setíferus y P.
duorarum. La mayor concentración de juveniles de
camarón blanco se localiza en el suroeste de la Laguna de
Términos. Se estima una biomasa de camarón mensual en este sector
de 0.268 + 0.163 gm -² con una eficiencia de arrastre de 0.4.P.
setíferus contribuye con casi 90% a esta biomasa
estimada.
The distribution and abundance of the
estuarine stages of the populations of Penaeus duorarum, P.
setíferus and P. aztecas in Terminos
Lagoon is given. Immigration of planktonic postlarvae into the lagoon system
occurred throughout the year, though peak values were detected in Julio and
November. The two main species which entered as postlarvae were P.
setiferus andP. duorarum. Juvenile of the three
species presented a distint spatio-temporal distribution in the nursery ground.
Brown and white shrimp showed a temporal exclusion in the soft bottom habitat
found in the southwestern area of the lagoon. Pink shrimp exhibited a spatial
segretation, as its main distribution was located on vegetated calcareous
substrates in the eastern area. Two periods of low and high shrimp abundance
were recognized, with higher variability in the earlier stages. In an annual
cycle two main cohorts of P. seliferus and P.
duorarum emigrating from Terminos Lagoon were identified. The major
concentration of juveniles was found in the western sector, which is enriched
by river discharge. Estimated monthly shrimp biomass of the southwestern sector
of lagoon with a trawl efficiency of 0.4 was 0.268 + 0.163 gm-²
P. setiferus contributed nearly 90% of this
biomass.
Traditionally, estuaries have been recognized as highly productive systems which are essential to fishery production (Turner, 1979; Waldichuck, 1979; Boesch and Turner, 1984). Penaeid shrimps constitute a vital renewable resource whose early life cycle is intimately connected to the condictions, that prevail in the estuarine environments and the wetlands that border the Gulf of Mexico. The critical function that these systems represented to the shrimp stocks harvested in the offshore waters of the Gulf of Mexico has clearly been demonstrated in Texas, Louisiana, Mississippi and Florida (Kutkuhn, 1962, Baxter, 1963; Christmas et al., 1966; Subrahmanyam, 1971; St. Amant et al., 1962; Zimmerman and Minello, 1984). These habitats are areas of recruitment and growth for the shrimp postlarval and juvenile stages of the three major commercial species in the Gulf: Penaeus duorarum, P. setiferus and P. aztecus. In the southem Gulf of Mexico, the area known as Campeche Bank, represents and excellent habitat for a large number of commercial species which have an estuary dependent life cycle. It is estimated that approximately 90% of the commercial catch consists of coastal and estuarine species which spawn offshore, reach estuarine areas either as larvae or postlarvae, and remain there until they attain subadult size (Soto et al., 1981). Along the coastline of Campeche Bank, which extends nearly 400 nautical miles (780 km) from the state of Tabasco to the Yucatan coast, the largest embayment is Terminos Lagoon. The large surface area ( 2,500 km²) and high biological productivity of this mangrove seagrass dominated estuary provide and assorment of substrates for the settlement of shrimp postlarvae and adequate sources of food and cover. Until 1973, Terminos Lagoon supported a modest artisanal shrimp fishery largely concentrated on the juvenile shrimp. However, to prevent and adverse effect on the offshore fishery, México declared in 1974 that Terminos Lagoon would be a protected reservoir. This resolution, assumed that Terminos Lagoon was largely responsible for the supply of juveniles to the adult stock exploited along the Campeche Bank. The implications for future management strategies of this resolution made necessary a study of the population characteristics of the different stages of the shrimp populations inhabiting the lagoon. The Universidad Nacional Autónoma de México, through the Instituto de Ciencias del Mar y Limnología, is actively participating in the study of the living resources of Terminos Lagoon and the adjacent continental shelf (Soto, 1980). This paper describes part (August 1979-July 1980) of a three year program, whose objective is to characterize the patterns of movement, density and growth of the shrimp populations that utilize Terminos Lagoon as a nursery ground. STUDY AREATerminos Lagoon is situated along the coast of the southern Gulf of Mexico, adjacent to Campeche Bank, (91º15-91º51' W and 18º27-18º50' N) with an area of about 2,500² km . The lagoon is separated from the Gulf by Carmen Island, but maintains communication with the marine environment through two wide inlets (Fig. 1). Puerto Real Inlet to the northeast of Carmen Island is 3.3 km wide and has a maximum depth of 15 meters. Carmen Inlet to the southwest of the island is 4.2 km wide and has a maximum depth of 11 meters (Cruz-Orozco, 1980). The lagoon is shallow, with a mean depth of 2.5 meters and maximum depths at the center of the basin (Yáñez, 1963). Due to the prevaling easterly winds, there is a net inflow of salt water into the lagoon through Puerto Real Inlet and a net outflow of water to the Gulf through Carmen Inlet (Phleger and Ayala-Castañares, 1971). Terminos Lagoon receives the discharge of several rivers into the westem part. The most important of these are the Palizada, the Chumpan and the Candelaria. The climate of the area is humid-tropical with strong storms provoked by northerly winds during the months of November to February (storm season). Maximum air temperature in summer is 36ºC and in winter is 17ºC (Toral, 1971). Lowest water temperatures are recorded in winter (24ºC) and the highest in Summer (32ºC). Annual precipitation ranges from 1200 to more than 2000 mm, with a maximum in the months of June to November (rainy season). Closely related to the highest rainfall is the period of high river flow that occurs from August to November. The dry season extends from February to May. The salinity fluctuations in Terminos Lagoon are directly related to the precipitation regime. The highest salinity values 35‰ are found near Puerto Real and Carmen Inlets, and salinities decrease to 2‰ towards the areas affected by the runoff from the rivers. The lagoon has a mangrove shoreline. Bottom substrate in the southeast and inner margin of Carmen Island is covered with seagrass. Mud bottom in the southwestern is naked. Tides are mixed diurnal with a maximum amplitude in the year of 0.5 m (Grivel and Arce, 1975). More detailed information on the physiographic features of the lagoon can be found in Yañez (1963), Phleger and Ayala-Castañares (1971), and Mancilla and Vargas (1980). MATERIAL AND METHODS
PLANKTONIC POSTLARVAEPlanktonic Postlarvae were sampled twice a month at Puerto Real Inlet from July 1979 to July 1980. A flood tidal delta in this inlet is cut by channels of various depths. The channel with the maximum depth (~15 m) was chosen to, position two cylindrical plankton nets, one at the surface and the other near the bottom. The sampling was conducted weather permitting, every two, hours, for more than 12 hours extending well into the night. To observe diel and lunar rhythms the dates of sampling were selected according to periods of maximum tidal range (i.e. during new moon and full moon). The nets employed were 1.5 m in length, 0.5 m in diameter, had a mesh size of 450μ and a filtration efficiency above 85%. The volumen of water filtered, through the nets was recorder by digital flow meters secured at the mouth of each net. Surface and bottom readings of temperature and salinity were also made every two hours with a salinometer. Postlarvae were recorded in terms of number of postlarvae per hour and per 100 m3. Identification of postlarvae was carried out with, the aid of the generic and specific characters described by Cook (1966), Pearson (1939), Williams (1959), and Ringo and Zamora (1968). JUVENILE STAGESFor a year beginning in August 1979, juvenile shrimp were sampled monthly (excluding December, 1979 and January, 1980) at 18 localities distributed along 5 transects from Carmen Island to the inner margin of the lagoon (Fig. 1). The sampling gear was a small otter trawl of 5 m head rope lenght with 2.5 cm stretched mesh. Estimated area swept during a 12 minute trawl was. 1,852 m2. Observed biomass in a sample was converted to estimated standing crop using a 40% mean efficiency value (Loesch et al., 19176; Edwards, 1977). All trawling in the lagoon was done during daylight hours. In the laboratory shrimps were sorted according to species, and sex, and then were weighted (0.1 g) and measured (0.1 mm Carapace length). 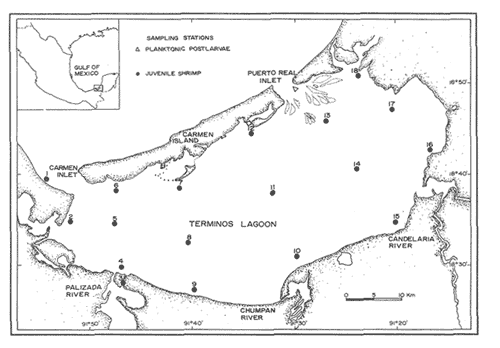 Figure 1. Location of the study arca and sampling stations RESULTS
RECRUITMENT PATTERS
Planktonic PostlarvaeThe total number of shrimp postlarvae captured was 5,872. Four genera of penaeid postlarvae were identified over the year of sampling at Puerto Real Inlet (Table 1).The postlarvae belonging to the genus Penaeus clearly predominated over the other three genera whose occurrences were occasional and which represented only a small fraction of the total number of postlarvae collected by Arenas and Yáñez (1981). The information obtained in this study suggest that none of these genera(Trachypenaeus, Sicyonia andXiphopenaeus) utilize the lagoon as a nur sery ground. In contrast, the species Penaeus setiferus and P. duorarum appeared frequently in all the samples. P. Setiferus comprised 80% of all postlarvae (4,743 individuals), whileP. duorarum represented 14% (817 individuals). The absence of postlarvae of P. aztecus, normally captured as adults in the adjacent offshore waters, was not expected. Planktonic postlrvae were always present in the water column at Puerto Real Inlet; however fluctuations in the immigration pattern, were atributed to the prevailing weather regime. The major influx of postlarvae took place during the rainy season, reaching peaks in July and November 1979, and June 1980 (Fig. 2). Maximum numbers of P. setiferus were 45/100 m³ in July and 42/100 m³ in November. Highest values for P. duorarum were 8/100 m³ in July and 16/100 m³ in November. A moderate immigration was also registered early in February 1980 during the dry season, though the number of postlarvae was generally low (Table 2). The mean densities of the above two species during the dry season in the early part of the year substantially diminished (from 0.34 to, 14/100 m³) and it was not until late in June that important recruitment of postlarvae was again detected. 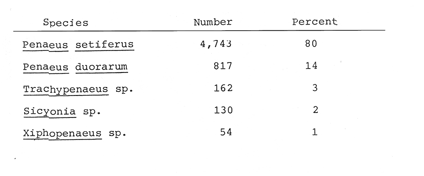 TABLE 1 SPECIES COMPOSITION AND RELATIVE ABUNDANCE OF PLANKTONIC POSTLARVAL SHRIMP CAPTURED AT PUERTO REAL INLET, TÉRMINOS. 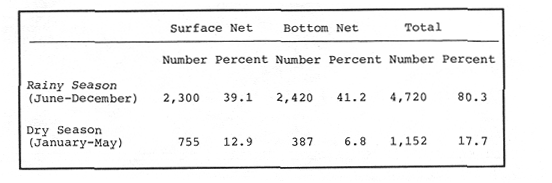 TABLE 2 SEASONAL DISTRIBUTION OF PLANKTONIC SHRIMP POSTLARVE AT PUERTO REAL INLET, TERMINOS LAGOON One factor that may condition the position of the postlarvae in the water column at the timie of entrance is the diel rhythm. In the case of P. duorarum the onset of darkness or cloudy conditions seemed to trigger the influx of postlarvae, preferably along the bottom. Immigrant postlarvae of P. setiferus penetrate into the nursery area at day or night without showing significant vertical stratification. 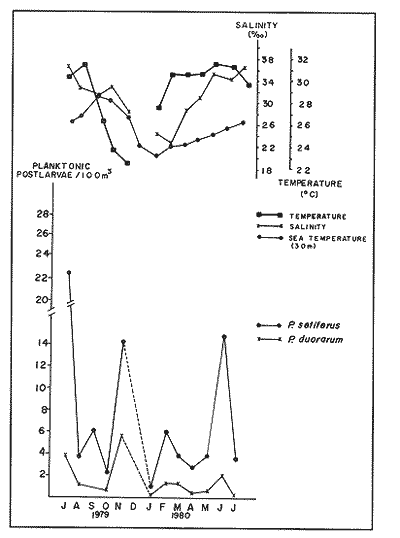 Figure 2. Annual ocurrence of penaeid planktonic postlarvae in Términos Lagoon. Abundance is referred to number of postlarvae by 100 m3 of filtered water. JUVENILE STAGES
Distribution and size patterns
P. setiferusThis species occurred in Terminos Lagoon all year around. Its abundance was correlated (r = 0.73, P<0.02) with the precipitation regime of the area studied (10 to 370 mm). Largest catches were obtained from June through November, with a maximum value recorded during the latter month (Fig. 3). The conditions prevailing in the dry season, high salinities (22 to 34%) and low precipitation values (10 to 45 mm) were related to a considerable decrease in monthly catches of white shrimp. The juvenile population of P. setiferus appeared to be largely concentrated towards the southwestern sector of the lagoon where the effect of the river's discharge is felt more strongly than in any other portion of the area studied (Fig. 6). This pattern of distribution is corroborated by the progression of the monthly mean sizes of juvenile shrimp recently reported by Aguilar(1985), whose data clearly indicated an increase in size of individuals sampled as they move towards the Carmen Inlet. The total lenght of the white shrimp captured ranged from 25 to 155 mm, with monthly mean sizes of 67 to 103 mm (Fig. 4). Periods of recruitment of P. setiferus to the juvenile population inhabiting Terminos Lagoon are essentially observed during the months of precipitation, particulary towards the end of the fall season. The strong influx of planktonic postlarvae of white shrimp recorded in September produced a peak of juvenile shrimp in the lagoon two months later (Fig. 7). In spite of the void of information in December (Fig. 3) the growth rate ofP. setiferus estimated by mark-recapture (Gracia and Soto, 1986a) indicated that it takes roughly two months for a postlarvae to reach juvenile size (52 mm T.L.) Unfortunately, no data were collected on the abundance of juvenile P. setiferus for the months of December and January, preventing assesment of the progress of the cohort of postlarvae detected in November. During the dry season, the time elapsed between peak values of postlarvae and of juveniles seems to be longer than two months. This implies that the residence time of the white shrimp within the lagoon could he extended up to five months in the dry season, whereas during the rainy season their residence is slightly shorter, from 2 to 4 months (Fig. 7). This was recently corroborated by Aguilar (1985) who estimated the residence time of the estuarine population of P. setiferus. The individuals of this species appear to leave their estuarine habitat when they reach mean total lengths of 80 mm. 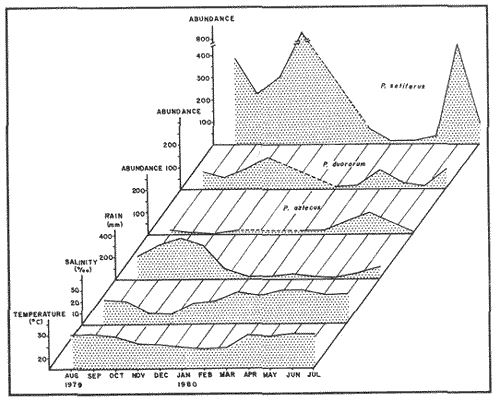 Figure 3 Composition of Penaeus setiferus, Penaeus duorarum and Penaeus aztecus. 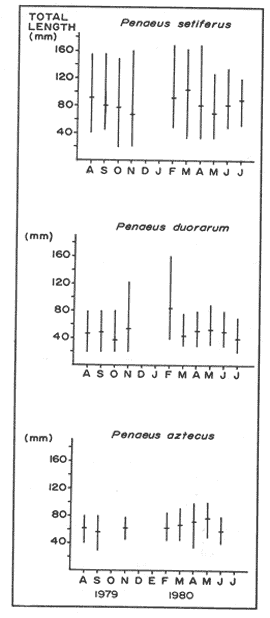 Figure 4. Monthly size composition of Penaeus setiferus, Penaeus duorarum and Penaeus aztecus. 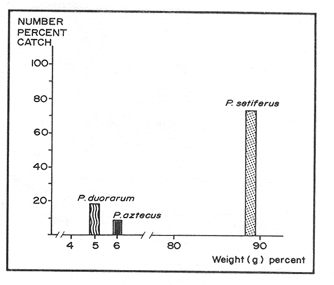 Figure 5. Species composition of Penaeid shrimp by weigth and number percentages during August-1979 July-1980 in Términos Lagoon. P. duorarumThe seasonal occurrence of pink shrimp in Términos Lagoon followed a trend similar to the already described forP. setiferus (Fig. 3). Although the monthly catches of P. duorarum were never as high as for white shrimps, its relative abundance did show a significant correlation (r=0.67, 0.05>P> 0.02) with the precipitation regime of the area studied. The maximum number of individuals (144) of this species was captured in November. The juvenile of P. duorarum appeared widely distributed in the lagoon and in some areas like the southwestern sector its distributional pattern averlaps with that of P. setiferus. However, the most significant concentrations of pink shrimp were found in the area just off the Candelaria River and in the proximity of Puerto Real Inlet (382 individuals). 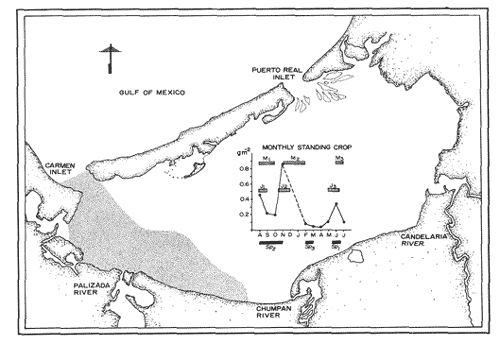 Figure 6. Area of major concentration of Penaeus setiferus (shaded) and estimated mean standing crop in the soutb-westem sector of Términos Lagoon. Occurrence of apawning (Sp), juvenile (J), and migration (M) of the main cohorts. The range of total length ofP. duorarum (20-160 mm) did not differ much from that recorded for the white shrimp (Fig. 4). However, the average size tended to be smaller as revealed by their monthly means (39 to 82 mm T.L.). The mean size did not differ from month to month and the predominance of small sizes suggestes the occurrence of continuous recruitment throughout the year, though recruitment was always more intense during the period of rainfall. No differences in size (P>0.05) composition were observed between the pink shrimp populations captured in dry and rainy seasons. From figure 7 it can be inferred, that it takes from 6 to 8 weeks for this species to reach the juvenile stages (34.8-46 mm T.L.). After this period the juvenile shrimps remain in the lagoon aproximately a month before they move out to offshore waters. P. aztecusP. aztecus were the least abundant juveniles of the three commercial species. The number of brown shrimp collected during the year was low (268) and only a small peak was registered in the spring between May and June (Fig. 3). The highest number of juvenile ofP. aztecus was recorded when the number of individuals of white shrimp was low. The distribution of the few juveniles collected was limited to the southwestem portion of Terminos, Lagoon and particularly near Carmen Inlet, where the higher overall concentrations of juveniles were found. The size range of P. aztecus (30 to 100 mm T.L) inside the lagoon was narrow compared with those of P. setiferus and P. duorarum (Fig. 4). Apparently the brown shrimp enter the estuarine system only as a juvenile stage and remain there for a short time. The juvenile of P. aztecus seem to leave the lagoon when they attain a mean size of 80 mm T.L. 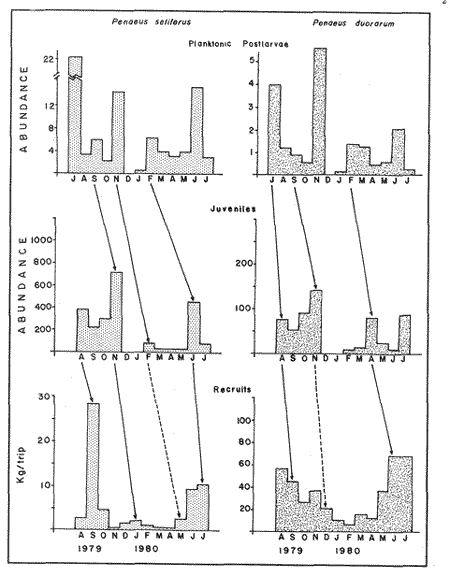 Figure 7. Sequence of the main annual cohorts of Penaeus setiferus and P. duorarum from planktonic to recruits Solid and broken lines indicate the progression of major cohorts Offshore catch record of P. setiferus and P. duorarum obtained from Secretaria de Pesca. JUVENILE SHRIMP BIOMASS PATTERNP. setiferus represents 74% of the total number of shrimp captured throughout the year, whereas, P. duorarum and P. aztecus constituted only 18% and 8%, respectively. The biomass of P. setiferus contributed nearly 90% to the annual juvenile shrimp catch (12,300 g), while biomass, values of the other two species were negligible (Fig. 5). Juvenile Sicyonia sp. and Trachypenaeus similis do not seem to use the lagoon as a nursery ground and their capture was infrequent. Only X. kryoyery showed some sporadic abundance in the area just off the Carmen Inlet. Maximum values of shrimp biomass were obtained at sampling station 2 (Fig. 1). Maximum biomass recorded in a trawl at this size were 1.58 and 5.38 gm-² in August and November, respectively. Station 1 at Carmen Inlet also registered a high value (1.72 gm-²) in June. In the southwestern area, stations 8 and 10 had the lowest shrimp yield. Monthly mean shrimp biomass followed a similar trend to that of numbers of P. setiferus (Fig. 6). Monthly mean biomass with 95% confidence intervals estimated in the entire lagoon was on the order of 10.104 ± 0.069 gm-² wet weight. P. setiferus contribute 89% to the total catch, whereas P. duorarum and P. aztecus constituted only 6% and 5%. However, this biomass may be overstimated since 95% of the total juvenile catch obtained in the southwestern sector between the mouth of Chumpan River and Carmen 2 Inlet (Fig. 6). In this area of approximately 380 km², shrimp biomass was mainly based onP. setiferus and amounted to a monthly average of 0.268 ± 0.163 gm-². Due to the nature of the sampling technique employed in this study, confidence intervals should be considered as indicatives. 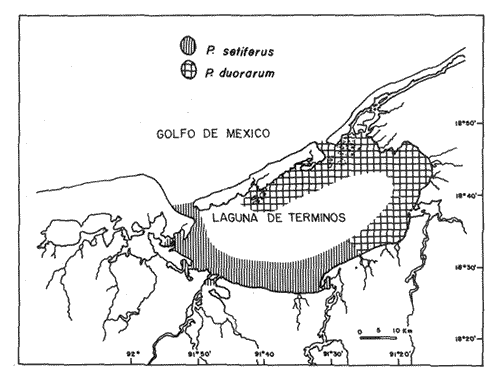 Figure 8. Main dístribution of Penaeus setiferus and P. duorarum in Términos Lagoon. P. aztecus is found in the same área of P. setiferus at different time. DISCUSSION AND CONCLUSIONSThe ecological importance of Terminos Lagoon as a recruitment area for a number of species, and its role as a vital source of nutrients to the adjacent marine environment has been widely recognized (Signoret, 1974; Soto et al, 1981; Yáñez-Arancibia and Day, 1982). The number of recruits to the shrimp fishery is regulated through a combined mechanism which includes biotic and abiotic factors. Seasonal variations in the carrying capacity of the lagoon affect strongly the level of postlarval settlement, whereas density dependent conditions act upon shrimp cohort characteristics (Gracia and Soto, 1986b). Several authors (St. Amant et al, 1962; Kutkuhn, 1962; Baxter, 1963; Yokel et al, 1969) have indicated the convenience of determining the movement and density of postlarval stages as feasible way of forecasting the success of commercial shrimp on the offshore fishing grounds. In general terms, the annual influx of postlarvae entering Términos Lagoon through Puerto Real Inlet fits quite closely the spawning periods recognized in the northern Gulf of México, forP. setiferus, P. duorarum and P. aztecus (Temple and Fisher, 1967; Pérez-Farfante, 1969; Subrahmanyam, 1971). The continuous immigration of planktonic postlarvae in the area suggests a protracted spawning activity for P. setiferus andP. duorarum, as it has also been observed for other tropical penaeids (García and Le Reste, 1981). The postlarvae of the above two species follow a similar recruitment trend, though the pulses of P. duorarum were not as a strong as those ofP. setiferus, and were only intensive during the fall season. The annual influx of postlarvae of P. duorarum is similar to that described by Allan et al. (1980) for this species in the Florida Keys; however, the peak values reported by these authors are considerably greater than those obtained in the present study. Arenas and Yáñez (1981) demonstrated that the immigration of postlarvae through the Puerto Real Inlet takes place within an ample range of salinity, 18-38%, and that the optimum influx can be observed at temperatures of 26.8° - 28.7ºC. The maximum immigration pulses recorded in June, July and November appear asociated with an increase of bottom temperature in offshore waters (26-28.5ºC) from June through November (Fig. 2), which undoubtedly promotes spawning activity of adult shrimp. In spite of their reduced number in the plankton, the density values of early juveniles of P. duorarumrecorded on the eastern sector were higher or aqual than those of P. setiferus. This is attributed to the substrate preferences exhibited by both species. Pink shrimp settle on calcareous sediments covered by turtle grass, while white shrimp select muddy substrates rich in organic matter found in the southwest of the lagoon. According to Zimmerman and Minello (1984),P. setiferus may settle either on marsh vegetation or adjacent nonvegetated bottom in Galveston Bay. In Terminos Lagoon white shrimp also settle on both substrates (Gracia, 1989a). However, the white shrimp population is concentrated in the southwestern area where a bare soft bottom is found. The virtual absence of brown shrimp postlarvae could indicated that P. aztecus makes only a limited use of the lagoon as nursery ground. However, it should be pointed out the considerable difficulty in distinguishing the postlarvae and early juveniles of this species from those belonging to P. duorarum. This problem has also been conceded by Stokes, (1974) in his study made in the lower Laguna Madre of Texas. The taxonomic characters proposed by Pearson (1939) and Williams (1959) show ample variations and therefore are not entirely reliable. Future efforts should be directed to examine the applicability of other possible characters, like those discussed by Mair (1979) and Cabrera-Jiménez (1983), that may increase the accuracy in the identification of penaeid shrimp postlarvae. It should be noted that presence of P. aztecus epibenthic postlarvae was registered in a small lagoon adjacent to Terminos Lagoon during November December (Hernández et al, 1987). The occurrence of P. aztecus in the Terminos Lagoon in this study was restricted to few juvenile shrimp captured mostly during the spring when waters in the lagoon were essentially euryhaline. The movements of penaeid shrimp into and out inland waters have been atributed among others things, to the osmoregulatory capacity of the individuals. The presence of P. aztecus through the year seems to coincide with a marked reduction in the number of white shrimp entering the lagoon from March to May (Fig. 3). This type of behaviour between P. aztecus and P. setiferus has also been observed in Galveston Bay (Chin, 1960; Baxter, 1963; Berry and Baxter, 1969; Parker, 1970). It is clear that Terminos Lagoon serves at one time or another, as a nursery ground to four species of penaeids. However, it is remarkable the way these species distribute themselves in time and space to avoid competitive interactions and to ensure the survival of their recruits. Thus for instance, P. setiferus, present throughout the year, is the dominant species from June onwards (rainy season), and co-occurs incidentally with the seabob Xiphopenaeus kryeri on the western end of the lagoon. In contrast, as pointed out before, there could be a temporal exclusion between juveniles of P. aztecus andP. setiferus possible due to the hydrological condictions prevailing during the spring and early summer (dry season). The white and pink shrimp populations, although exhibiting similar monthly fluctuations, are spatially segregated (Fig. 8). The euryhaline condition ofP. setiferus enables it to succesfully invade the estuarine habitat, thus allowing the individuals to settle in a much wider area.P. duorarum, in turn, displays a distribution restricted to areas of polyhaline waters, normally detected along the inner margin of El Carmen Island and at the proximity of Puerto Real Inlet; in addition to this, its residence time the lagoon is shorter than that of P. setiferus. The evidence demonstrates the key role that Terminos Lagoon provides for early stages of the white shrimp; and pink and brown shrimp, which sustain an offshore fishery, also use the lagoon to a lesser known extent. Even though the recruitment pattern of early stages of white and pink shrimp is almost continuous through the year, two distinct periods of low and high abundance can be recognized. García (1985) has suggested a general double peaked pattem for shrimp spawning activity, which results in a bimodal annual fishery recruitment cycle as the most frequent behavior in shrimp populations. The shrimps of southwestern Gulf of Mexico somehow conform to this pattern in terms of abundance, though their spawning activity seems to have bimonthly pulses (Gracia and Soto, 1986b). Disregarding sampling variablility that may reduce the significance of the monthly abundance values, three main cohorts can be identified in the lagoon: two in the high abundance period (June to November) and one in that of low abundance (February to May, Fig. 7) Each cohort had its own characteristics (abundance, growth rate and residence time) and corresponded to the three climatic seasons known in the area of study (windy-rainy-dry). Additional evidence of this relationships was recently offered by Smith (1984), Aguilar (1985), and Alvarez et al. (1987). Catch records of the offshore trawl fishery depict two recruitment peaks during the year (Fig. 7), which only support the existence of two main cohorts in an annual cycle, thus suggesting two main migration periods out of Terminos lagoon (Fig. 7). The influence of rainfall on shrimp populations has been documented by many authors in different species (Ruello, 1973; Castro-Aguirre, 1976, Castro Ortiz and Sánchez Rojas, 1976; García and Le Reste, 1981; Gracia, 1989b). Rainfall, through the resultant freshwater runoff, may promote primary productivity (Day et al., 1982) and the food supply in Terminos Lagoon, which in turn benefits recruitment, survival and shrimp growth. The monthly mean biomass estimated in the southwestern sector is comparable to other values (0.097 - 0.826 g m-²) recently obtained in the lagoon for P. setiferus by Aguilar (1985). Also maximum mean monthlyl shrimp biomass registered in August (0.473 gm-²) and November (0.877 gm-²) were similar to that obtained for P. setiferus (0.62 ± 0.3 g m-²) by mark-recapture tectiniques in a small creek adjacent to Terminos Lagoon during the rainy season (Gracia and Soto, 1986a). Nonetheless, these values are considerably lower than monthly maximum obtained for temperate salt marshes in Southern Louisiana (1.45 - 3.27 g m-²; Jacob, 1969) and shrimp catch (up to 155 tons per month) reported for Mobile Bay (Loesch, 1976). Likewise such values are inferior to the production estimated (10 to 11 g m-² 4 to 5 months-¹) in the tropical system of Huizache-Caimanero lagoon, on the northwestern Pacific coast of Mexico (Mendoza Von Borstel, 1972; Edwards, 1977). However, maximum values recorded in station 2 in this study (1.58 - 5.38 gm-²) indicate standing crops in certain areas of the lagoon similar to that of temperate estuaries which sustain an inshore fishery. A more accurate estimation of Terminos Lagoon's shrimp population biomass is required in order to assess the potential of this resource and its proper management. Preliminary calculations based on the trawl data of this study, offer a tentative figure of roughly 102 tons per month of shrimp standing crop. The lack of catch statistics on the artisanal fishery not only precludes the evaluation of the resource but emphasizes the need to further study the shrimp inhabiting Terminos Lagoon. AgradecimientosFunds for this research were made available by the Consejo Nacional de Ciencia y Tecnología (CONACyT) and instituto de Ciencias del Mar y Limnología, UNAM through the project PCMANAL-790334. The continuous support of the personnel included in this projects is greatly appreciated. LITERATURAAGUILAR-SIERRA, V. A.Tesis Prof. Camarones peneidos de la Laguna de Términos, Campeche: Composición, Distribución y Parámetros Poblacionales. Fac. de Ciencias, Univ. Nal. Autónoma de México1985. 62 p. ALLEN, D. M., H. HUDSON and T.J. COSTELLO Bull. Mar. Sci. Postlarval shrimp (Penaeus) in the Florida Keys: species, size and seasonal abundance. 1980. 21-33.30 (1): ALVAREZ, F., A. GRACIA and L. A. SOTO An. Inst. de Cienc. del Mar y Limnol. Univ. Nal. Autón. de MéxicoCrecimiento y Mortalidad de las Fases Estuarinas del camarón rosado Penaeus Farfantepenacus duorarum Burkenroad 1939 en la Laguna de Términos, Campeche.1987.207-220.14 (2): ARENAS-MENDIETA, M R. and A. M. YAÑEZ Tesis Prof. Patrón anual de inmigración de postlarvas de camarón (Crustacea: Decapoda: Penaeidae), en la Boca de Puerto Real, Laguna de Términos, Campeche.Fac. de Ciencias, Univ. Nal. Autón. de México1981. 92p. BAXTER, K. N. Abundance of postlarval shnmp one index of future shrimping success.Proc. Gulf Caribb. Fish. Inst.1963.79-81. 15: BERRY, R. J. and K. N. BAXTER Fish. Rep.Predicting brown shrimp abundance in the northwestern Gulf of México. FAO1969. 775-798. (57)Vol. 3 BOESCH, D. F. and R. E. TURNEREstuaries Dependence of fishery species on salt marshes: The role of food and refuge.1984.460-468. 7 (4A) CABRERA JIMENEZ J. A. Crustaceana Characters of taxonomic value of the postlarvae of the shrimp Penaeus Farfantepenaeus brevirostris Kingsley (Decapoda, Natantia), of the Gulf of California, México. 1983.292-300.44 (3) CASTRO AGUIRRE, J. L. Mem. Simposio sobre Biología y Dinámica Poblacional de Camarones.Guaymas Son. MéxicoEfecto de la temperatura y la precipitación pluvial sobre la producción camaronera. In: J. L. CASTRO-AGUIRRE (Ed.). 1976. 74-88.1 8 al 13 de agosto de 1976 CASTRO ORTIZ, J. L. and M. A. SANCHEZ ROJAS Mem. Simposio sobre Biología y Dinámica Poblacional de Camarones. Notas preliminares del comportamiento y Dinámica Poblacional de Penaeus stylirostris Stimpson, 1971, en los sistemas lagunares del centro de Sinaloa. Guaymas, Son. México In: J. L. Castro Aguirre (Ed.) 1976.213-254. 11: 8 al 13 de agosto de 1976 CHIN, E. The bait Shrimp Fishery of Galveston Bay, Textas. Trans. Am. Fish. Soc.1960.135-141.89 (2): CHRISTMAS, J. Y., G. GUNTER and P. MUSCRAVE Studies of annual abundance of postlarval penaeid shrimp in the estuarine waters of Mississippi as related to subsequent commercial catches.Gulf Res. Rep. 1966.177-212.2 (2): COOK, H. L. Fish. Bull. A generic key to the protozoean, mysis and postlarval stages of the lioral penaeidae of the northwestern Gulf of México. 1966. 437-447.65 (2): CRUZ-OROZCO, R, Tercer Reporte, presentado al Consejo Nacional de Ciencia y Tecnología Estudio del sistema fluvio-lagunar deltaico de la Región de Campeche, Tabasco, en particular de la Laguna de Términos y áreas adyacentes, para su mejor uso y aprovechamiento.México. 1980. 61 p. DAY, J. W, R.H. DAY, M. T. BARREIRO, F. LEY LOU and C.J. MADDEN Coastal Lagoons Oceanol. Acta Vol. Spec. Primary Production in the Laguna de Términos, a tropical estuary in the southern Gulf of México. In: P. Lasserre and H. Postma (Eds.). 1982.269-280.5 (40) EDWARDS, R. R. C. Esturaine and Coastal Marine Science Field experiments in growth and mortality of Penaeus vannamei in a mexican coastal lagoon complex.1977.107-121. 5 GARCÍA, S. Second Australian National Prawn Seminar, NPS2, Reproduction, stock assessment models and population parameters in exploited penaeid shrimp populations. In: ROTHLISBERG, P. C., B. J. HILL and D. J. STAPLES (Eds.). Cleveland Queensland Australia:1985.139-158. GARCÍA, S. and L. LE RESTE Life cycles, dynamics, exploitation and management of coastal penaeid shrimp stocks. Fish. Tech. Pap.FAO1981. 215 p. (203) GRACIA, A. Tesis Doctoral Ecología y Pesquería del camarón blanco Penaeus setiferus (Linnaeus, 1767) en la Laguna de Términos-Sonda de Campeche.Fac. de Ciencias, Univ. Nal. Autón de México1989a.127p. GRACIA, A. An. Inst. de Cienc. del Mar y Limnol. Univ. Nal. Autón. México Relationship between environmental factors and white shrimp abundance in the southwestern Gulf of México. 1989b.171-182.16 (2): GRACIA, A. and L. A. SOTO An. Inst. de Cienc. del Mar y Limnol. Univ. Nal. Autón. de México Estimación del tamaño de la población, crecimiento y mortalidad de los juveniles de Penaeus setiferus (Linnaeus, 1767) mediante marcado-recaptura en la La Laguna Chacahito, Campeche, México.1986a.217-230.13 (3): GRACIA, A. and L. A. SOTO Workshop on Recruitment in Tropical Coastal Demersal Communities. IOC Workshop Report Condiciones de reclutamiento de las poblaciones de camarones peneidos en un sistema lagunar-marino tropical: Laguna de Términos-Banco de Campeche. In: A.YÁÑEZ-ARANCIBIA y D. PAULY (Eds). IOC/FAO1986b.257-265. No. 44: GRIVEL, P. F. and R. ARCEAn. Inst. Geof. Univ. Nal. Autón. MéxicoConfiguración cotidal de la Laguna de Términos, Campeche. 1975. 139-144. 21 HERNÁNDEZ, G. A., A. GENIS, A. GRACIA and L A. SOTOMem. IX Congreso Nacional de Zoología Densidad y estructura poblacional de P. setiferus en la región suroccidental de la Laguna de Términos, Campeche.Villahermosa, Tab.1987. 13-16 de octubre de 1987. JACOB, W. J. M: S. Thesis Observations on the distribution growth, survival and biomass of juvenile and subadult Penaeus aztecus in Southem Louisiana. Louisiana State University:1969.1-68. KUTKUHN, J. H. Expanded rescarch on the Gulf of México shrimp resources.Proc. Gulf Caribb. Fish. Inst. 1962.65-78.15 LOESCH, H. C Shrimp populations densities within Mobile Bay. Gulf Res. Rep. 1976. 11-16. 5 (2) LOESCH, H. C., J. BISHOP, A. CROWE, R. KUCKYR and P. WAGNER Techniques for estimating trawls efficiency in catching brown shrimp (Penaeus aztecus), Atlantic Croaker (Micropogon undulatus) and Spot (Leiostomus xanthurus). Gulf Res. Rep.1976. 29-33. 5(2): Mac FARLAND, W. N. and B. D. LEEBull. Mar. Sci. Gulf and Caribb. Osmotic and ionic concentrations of penaeidean shrimps of the Texas Coast. 1963.391-417. 13: MAIR, J. M. J. Zool. Lond The identification of postlarvae of four species of Penaeus (Crustacea: Decapoda) from the Pacific Coast of México. 1979. 347-351.188. MANCILLA PERAZA, M. and M. VARGAS FLORES An. Centro Cienc. del Mar y Limnol. Univ. Nal. Autón. México Los primeros estudios sobre la circulación y el flujo neto de agua a través de la Laguna de Términos, Campeche. 1980.1-12.7 (2): MENDOZA VON BORSTEL, X. Mem. IV Congreso Nacional de Oceanografía. Efectos de la marea sobre la producción camaronera en aguas litorales. In: J. Carranza (Ed.). D.F. México1972.407-418. Nov. 17-19, 1969 PARKER J. C. Distribution of juvenile brown shrimp (Penaeus aztecus Ives) in Galveston Bay, Texas, as related to certain hydrographic features and salinity. Contrib. Mar. Sci. 1970. 1-12. 15: PEARSON, J. C Bur. Fish., Bull. The early life histories of some American Penaeidae, chiefly the commercial shrimp Penaeus setiferus (Linn.). U. S. 1939. 491-73. PEREZ-FARFANTE, I. Fishery BulletinWestern Atlantic shrimps of the genus Penaeus. 1969.461-591.67 PHLEGER,.F. B. and A. AYALA-CASTAÑARES Bull Am. Ass. Petrol. Geol.Proceses and history of Terminos Lagoon México.1971.2130-2140.55 (2): RINGO, R. D. and G. ZAMORA Bull. Mar. Sci. A penaeid postlarval character of taxonomic value. 1968. 471-476.18 (2): RUELLO, N. V. Mar. Biol. The influence of rainfall on the distribution and abundance of the school prawn, Metapenaeus macleayi, in the Hunter River region(Australia).1973.221-228. 23 (3): SIGNORET, M. An Inst. Biol. Univ. Mal. Autón. México Abundancia, tamaño y distribución de camarones (Crustacea, Penaeidae) de la Laguna de Términos, Campeche y su relación con algunos factores hidrológicos. Ser. Zoología 1974. 119-140. 45, (1): SMITH, M. K P.h. D. Thesis Some Ecological determinants of the growth and survival of juvenile penaeid shrimp Penaeus setiferus (Linnaeus) in Términos Lagoon, Campeche, México, with special attention to the role of population density. Univ. of California Berkeley1984. 151 p. SOTO, L. A. Decapod crustacean shelf fauna of the Campeche Bank: Fisheries aspects and ecology. Gulf Caribb. Fish. Inst. Proc. 32th. Ann Sess. 1980. 66-81. Nov. 1979 SOTO, L A., A. GRACIA and A. V. BOTELLO Study of penaeid shrimp population in relation to petroleum hydrocarbons in Campeche Bank. Gulf Caribb. Fish. Inst. Proc. 33th Ann Sess. 1981. 81-100. Nov. 1980. St. AMANT, L. S., K. C. CORKUM and J. G. BROOM Proc. Gulf Caribb. Fish. Inst. Studies on growth dynamics of the brown shrimp, Penaeus aztecus, in Louisiana waters. 1962. 81-100.33: STOKES, G. M. The distribution and abundance of penaeid shrimp in the lower Laguna Madre of Texas, with a description of the five bait shrimp fishery. Texas Parks & Wildlife Department Tech. Ser. 19741-13. 15: SUBRAHMANYAM, C B. Gulf Res. Rep The relative abundance and distribution of penaeid shrimp larvae off the Mississippi coast.1971291-339. 3 (2): TEMPLE, R. F. and C. FISCHER Fish. Bull. U.S. F. W. S. Seasonal distribution and relative abundance of planktonic-stage shrimp (Penaeus spp) in the northwestern Gulf of México. 1967. 323-33466 (22):1961. TORAL, S. Tesis Profesional Estudio de los Cichlidae de la Laguna de Términos y sus afluentes (Pisces: Perciformes).Fac. de Ciencias, Univ. Nal. Autón.México1971.32p. TURNER, R. E. Proc. Third Coastal Marsh and Estuarine Management SymposiumLouisiana coastal fisheries and changing environmental conditions. In: J. DAY JR., D. F. CULLEY JR., P. E. TURNER and A. J. MUMPHREY JR. (Eds.) L. S. U. Division of Continuing Education Baton Rouge, La 1979.363-370. WALDICHUK, M. In: Workshop on Coastal Area Development and Management in the Caribbean Región. IOC Workshop ReportEcosystem Protection in Coastal Area Development. Part 1. Classification of Coastal Areas.1979.1-29. No. 26 WILLIAMS, A. B. Bull. Mar. Sel Gulf and Carib. Spotted and brown shrimp postlarvae (Penaeus) in the North Carolina.1959.281-290. 9 (3) YAÑEZ, A. Inst. Geol. Bol. Univ. Nal. Autón MéxicoBatimetría, salinidad, temperatura y distribución de los sedimentos recientes de la Laguna de Términos, Campeche, México. 1963.47 p.67 (1): YAÑEZ-ARANCIBIA, A. y J. W. DAY, Coastal Lagoons Oceanologica Acta, Vol. Spec. Ecological characterization of Terminos Lagoon, a tropical lagoon estuarine system in the southern Gulf of México. In: LASSERRE, P. and H. POSTMA (Eds.). 1982.431-440. 5 (4) YOKEL, B. J., E S. IVERSEN and C. P. IDYLL Fish. Rep. Prediction of the success of commercial shrimp fishing of the Tortugas grounds based on enumeration on emigrants fron the Everglades National Park Estuary.FAO1969. 1027-1039. No. 57 ZIMMERMAN, R. J. and T. J. MINELLO Estuaries Densities of Penaeus aztecus, Penaeus setiferus, and other natant macrofauna in a Texas salt marsh. 1984.421-433. 7 (4)
|

