|
QUANTITATIVE STRUCTURE OF GORGONIAN
COMMUNITIES IN TAYRONA NATIONAL PARK, CARIBBEAN COAST OF COLOMBIA
Trabajo recibido el 10 de marzo de 1909 y aceptado para su publicación
el 25 de abril de 1990.
Leonor Botero
Instituto de Investigaciones Marinas de Punta de
Betín INVEMAR Apartado Aéreo 1016, Santa Marta, Colombia, South
America.
Las comunidades de
gorgonaceos del área de Santa Marta Parque Nacional Natural Tayrona se
describen aqui en terminos de su riqueza de especies (S), diversidad (H')
equitatividad (J') y densidad de colonias. Los índices de diversidad y
equitatividad variaron entre 0.7-2.4 y 0.4- 0.9 respectivamente, para las 9
estaciones muestreadas. Los índices de diversidad indican que los
gorgonaceos del Parque Nacional Tayrona estan en un ambiente predecible y
favorable ya que H' y J' son relativamente altos para todas las estaciones. No
se encontraron diferencias significativas de S, H' y J' entre las diferentes
estaciones. Tanto S como H' tienden a aumentar desde las zonas someras hacia
profundidades de 13-16 m. La maxima densidad encontrada fue de 6
colonias/m². Perfiles de zonación y analisis de agrupamiento de
las frecuencias de las especies indican que hay tres tipos principales de
comunidades de gorgonaceos en el área: aquellas localizadas entre 9 y 11
m de profundidad en los lados occidentales y agitados de ha ensenadas; aquellas
localizadas entre 0.5 y 18 m de profundidad en los lados orientales y
protegidos de las ensenadas; y aquellas localizadas entre 0.5 y 45 m de
profundidad en los lados protegidos de islotes.
Gorgonian
communities of the area of Santa Marta Tayrona National Park are described in
terms of their species richness (S), diversity (H') equitability (J') and
colony density. Diversity and equitability range between 0.7-2.4 and 0.4-0.9
respectively, for the nine stations sampled. Data and diversity indexes
indicate that gorgonians of Tayrona National Park are in a predictable and
favorable environment since both H' and J' are relatively high at all stations.
Values of S, H' and J' are not significantly different among the different
stations. Gorgonian S and H' generally increased from shallowest zones lo
depths around 13-16 m. Maximum density of colonice al any one site was 6
col/m². Zonation profiles and cluster analyses of species frequency data
indicate that there are three main gorgonian "community types" in the area:
thhose located between 9-11 m deep on western and agitated sides of bays, those
located between 0-5 and 18 m deep on eastern and protected sides of bays and
those located between 0.5 and 45 m deep on protected sides of islets.
In spite of the conspicuousness and abundance of gorgonians in Caribbean reefs, studies of their systematics, ecology and life histories are scarse, especially when compared to the large amount of information available on ecological, taxonomic and life-history aspects of Caribbean scleractinian corals. Community studies on species composition, distribution, zonation, diversity and abundance of gorgonian octocorals in different Caribbean locations have been made by Gonzalez-Brito (1970a, b), Kinzie (1970, 1973), Goldberg (1973a), Opresko (1973), Rees (1973), Preston and Preston (1975), Jordan and Nugent (1978), Jordan 1979), Alcolado (1981), Jordan et al (1981), Muzik (1982), Lasker and Coffroth (1983) and Botero (1987a, 1987b). Number of species known from these different locations ranges from 12 in Isla Margarita, Venezuela to 39 in Puerto Rico and 42 in Jamaica. Zooxanthellate gorgonians are reported for depths ranging from 1 to 55 m in the clearest waters. Their zonation seems to depend, according to some of these authors, on factors such as wave action, substratum and water transparency, and densities can be as low as 0.9 colonies/m² in certain areas around Jamaica and as high as 26 colonies/m² in coral patches close to Miami. Shannon Weaver diversity indexes range from 1 to 3.5 in the different Caribbean locations studied. Similar community studies in Australia and New Guinea are those of Tursch and Tursch (1982) and Dinesen (1983). Gorgonians of the Caribbean coast of Colombia had not been studied prior to the investigation of Botero (1987). Their species composition, horizontal distribution and vertical zonation in the area of Santa Marta and Tayrona National Park (TNP), were quantitatively described in Botero (1987a). She found 39 species, of which the majority of zooxanthellate ones are distributed between 1 and 18 m depth, since lack of hard, consolidated substratum inhibits their presence below these depths. Type of substrata available, amount of light and wave turbulence, are probably the main physical environmental factors that determine zonation and distribution patterns of gorgonians in TNP. This paper reports and analyzes information on density (# col/m²) and diversity of gorgonian communities in TNP from 0.5 m depth to limits of gorgonian growth (40-45 m) and compares communities of different sampling stations in terms of their species composition and frequency, density and diversity. The information obtained is compared with information available for other more typical Caribcean locations, where environmental conditions are stable throughout the year and thus somewhat different from those of the study area. DESCRIPTION OF THE STUDY AREATayrona National Park comprises a series of small, deep (0-70 m) inlets and bays as well as a 15 km stretch of more or less straight coastline (Fig. 1). Each of the inlets is diverse in types of environments, presenting coral- octocoral patches, Thalassia testudinum beds, seaweed beds, soft sand and mud bottoms, rocky subtidal and intertidal zones and sandy beaches. Hard substratum (rocky or coralline) is abundant for the first 10-15 m of depth along most of the coastline. The submarine relief resembles that of the mountainous topography of the adjacent land. From December to April the area is subjected to strong tradewinds blowing from the northeast. This creates upwelling in coastal waters, and surface temperatures drop lo 22°C and occasionally to 21°C. Turbulent waters and salinities greater than 36.5 ppt are typical for this time of the year (Bula- Meyer, 1977; Bula-Meyer, 1985; Ramirez, 1983; Salzwedel and Muller, 1983). Eastern sides of inlets and bays are generally more protected from wind action than western sides. In May there is a relative calm, and wind force increases again in June, July and August. From September to November (rainy season) the force of the trade winds decreases and the area is subjected to short term outwelling from the Magdalena River and Cienega Grande de Santa Marta whose brackish waters are carried eastward by the Colombian Countercurrent (Bula- Meyer, 1985). During this period, salinities can drop 2-5 ppt from upwelling values, and water temperatures are as high as 29°C. Waters are relatively calm and short periods of turbid surface waters are common. Normally, Secchi Disk readings vary between 17 and 28 m; however, occasionally during strong outwellings, Secchi Disk readings can be as low as 6-10 m. In more typical Caribbean locaties, temperature and salinity values are rather stable throughout the year and water transparency is much higher. 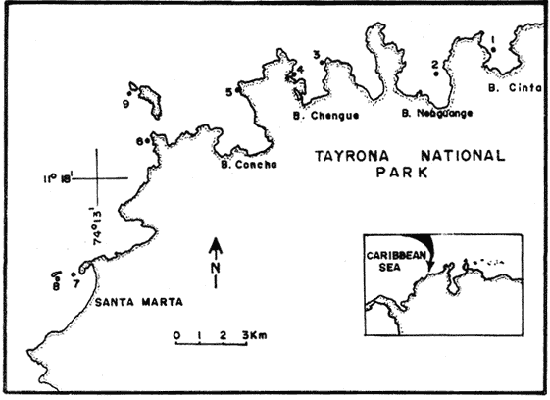 Figure 1. Par of the Caribbean Coast of Colombia showing the Santa Marta area and Tayrona National Park with its coastal inlets and bays. Numbers indicate location of the nine sampling stations. Dense gorgonian growth most commonly occurs on hard, steep substrata adjacent to the coastline, on shallow coral-octocoral patches in the middle of inlets, on and around big submerged rocks (30 m long x 15-20 m wide) whose tops emerge 2-3 m from the water surface (locally referred to as "piedras ahogadas", drowned rocks), and around islets. MATERIALS AND METHODSNine sampling stations were selected (Fig. 1) on the basis of previous observations on the presence of profuse gorgonian communities in the area and included exposed and protected sites of bays, inlets and islets and depths ranging between 0.5 m and 45 m. Only two stations were located on western sides of inlets because other inlets did not show profuse gorgonian growth on their western side. All field work and sampling was carried out by SCUBA diving between 0.5 and 50 m of depth by the author. Identification of gorgonian species was made using field characters (Cairns, 1977) and standard techniques of sclerite preparation (Bayer, 1961) from branches cut from living colonies. The minimum sampling area necessary to obtain significant results on the quantitative composition and structure of the gorgonian communities was determined by constructing cumulative species-area curves every 2 m of depth, using the method of Scheer (1978). At each depth interval, the necessary plot size was found to be 12 m horizontally x 5 m vertically (60 m²). However, to include a margin of safety, I extended this area to 75 m² (15 m horizontally = parallel to the coastline x 5 m vertically = perpendicular to the coastline) for sampling at different depth zones in Stations 2, 3, 5, 6 and 7. Depth limits of gorgonians at these stations were generally 13-14 m (20 22 m maximum in Stations 2 and 6) and it was found that 6 of these 75 m² rectangles, positioned one after the other, would include all depths at which gorgonians were present. Thus, total area to be sampled was equal to a rectangle 450 m² (15 m horizontally x 30 m vertically) divided into six 15x5 m horizontal strips (Fig. 3). Even though Stations 1 and 4 had no depth gradient, the same areal rectangle was sampled for comparative purposes. At Stations 8 and 9 gorgonians were distributed to greater depths (40-45 m) so that vertical length of the rectangle had to be extended to 84 m. All colonies within the plots were identified to the species level and their frequency of occurrence (number of individual colonies of each species per plot) was recorded. Data obtained from these counts were used for density (# col/m²) number of species (S) diversity (H') and equitability (J') determinations as well as for obtaining similarity indexes (Czekanowski Quantitative Similarity Index) (Bloom, 1981) to be used in cluster analyses of the data. Cluster analyses were carried out to help define zones at the different stations and the existence, or not, of defined "community types". RESULTSIn general, stations located on western sides of bays are exposed to strong wind action and present more turbulent waters than stations on eastern or protected sides of bays. (Table 1). On western or exposed sides, profuse gorgonian communities are found between 9-12 m depth on a relatively flat substratum made of hard rock platform covered by sand and/or flattened colonies of scleractinian corals. On eastern or protected sites, profuse gorgonian communities exist between 0.5-15 m depth on an approximately 20 slope substratum made of metamorphic rock and large and small coral heads. Stations located on the protected sides of islets have water agitation conditions similar to those of eastem sides of bays. However, average bottom slopes are much steeper (45-60°) and hard substratum reaches down to 35-40 m depth. Big rocks and scleractinian heads prevail down to 25-30 m, and from there down to 40 m, the bottom changes to unconsolidated cobbles and boulders intermingled with coarse sand. A detailed description of stations and of the zonation (quantitative profiles) of gorgonian species is given in Botero (1987a, 1987b). 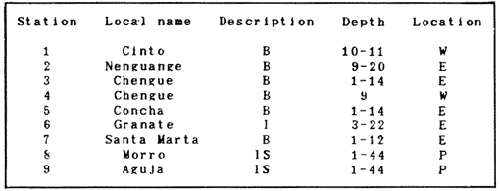 TABLE 1 STATIONS SAMPLED IN THE STUDY AREA; DEPTHS (IN M), AND EASTERN (E) OR WESTERN (W) LOCATION WITHIN THE BAY (B), INLET (I), OR PROTECTED (P) SIDE OF ISLET (IS) The quantitative structure of only five (Stations No. 1, 2, 3, 5, 9) of the nine sampled stations will be presented here in detail, because the general structural pattern of each of the other four (Stations No. 4, 6, 7, 8) is very similar to at least one of the five stations described and analyzed here, and it was already presented and discussed in Botero (1987a, b). Occassionally though, to clarify and give a better general idea of the structural patter of the communities of the area, references or comments will be made regarding these other 4 stations. Out of 38 species noted during the study (Table 2), the maximum number of species (S) ranged between 14 and 23 at one station (Fig. 7) and between 4 and 19 at any one depth zone (Figs. 3, 5, 6). In general, stations with pronounced depth gradients (Stations 2, 3, 5, 7, 8, 9) had highest S between 13-16 m depth. Density of colonies ranged between 0.6-6 col/m² figs. 2, 3, 4, 5, 6) (highest at Stations 5 and 9 between 6-8 m and 0-8 m depth respectively and lowest between 16 and 20 m depth a Station 2). Diversity (H') ranged from 0.7 in the deepest (18-20 m) zone of Station 2 (Fig. 3) to 2.4 in Stations 1 (Fig. 7) and 5 (at 12-14 m depth) (Fig. 5). Equitability (J') ranged from 0.4 at 9-10 m depth in Station 2 to 0.9 at 29-35 m depth in Station 8 and at 10 m depth in Station 1. When each station is considered as a whole (all zones pooled), Station 1 showed the highest J' value (0,8) (Fig. 7). This is depicted in the cumulative percentage curves for species (Fig. 2) at Station 1. They show clearly that the percentage frequency of occurrence of the two most abundant species (P. grisea and E. fusca) was never higher than 20% and that the 8 next most abundant species had rather similar frequency distributions, each species being no more than 5-10% of the community. Lowest J' occurred at Station 2, where only one species (E. fusca) made up 65-70% of the gorgonian population (Fig. 3). Stations with a depth gradient showed greater differences between J' values of the different depth zones of the rectangle (Figs. 3, 4, 5, 6) than did stations with no or a very slight depth gradient (Stations, 1, 4) (Fig. 2). This was expected since without a depth gradient very few changes in species frequency or dominance occur from zone to zone within the sampling rectangles. When a depth gradient exists, certain zones may show higher or lower predominance of a few species than others. At Station 3, G ventalina and P. flexuosa were predominant at shallower depths and thus J'was rather low while at greater depths the frequency distribution of species became more even and consequently J'increased (Fig. 4). At Station 5 there was an intermediate zone where M. flavida and G. ventalina made up almost 60% of the population and thus J'decreased from the value observed at shallower depths (Fig. 5). At Station 9, J'was high for the deepest zones even though only 4 species made up the gorgonian population (Fig. 6). The relationship between H'and J'and mumber of gorgonian species (S) recorded per depth zone (transect)and per station is presented in Figures 8 and 9. Although there is a general tendency toward increasing H'values as the number of species increases (r = 0.73, p<0.001, n =63), it is also apparent that often transects with higher "S" values may show lower heterogeneity (H'). This is because a transect or station could have a large number of species but their frequency distribution could be far from even; that is, a few species can be abundant while others, are scarse or, in general, the frequency distribution of species is extremely uneven. This is exemplified in Station 2, where S is larger than in Stations 1, 3, 4 and 6, yet H' is lower. The fact that J' is independent from S is depicted in Figure 9 (r = 0.04, p>0.05, n = 63) and is clearly shown in Station 9 (Fig. 6) where zones with lowest S showed highest J'. 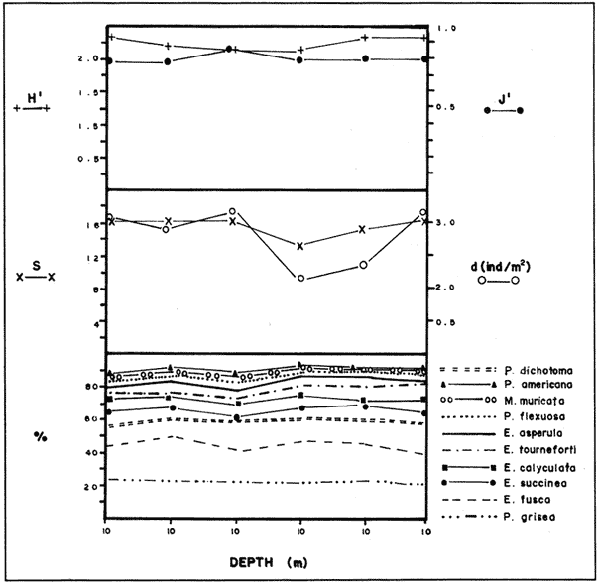 Figure 2. Data for the 6 zones of the sampling rectangle in station 1. Top graph: heterogeneity (H') and equitability (J') indexes. Middle graph: species richness (5) and density (d) of gorgonians. Bottom graph: cumulative percentage composition of most abundant species at cach of the zones. For Stations 3, 5, and 9, a zonation pattern determined by depth is clearly depicted in the dendrograms (Fig. 10). Zones within the sampling rectangle were grouped in direct relation to their depth, i.e. adjacent zones formed clusters. By combining the dendrograms with information provided by the quantitative zonation profiles (Botero, 1987b) it is evident that Station 3 has three major zones: 1) aG. ventalina P. flexuosa, M. Flavida Pseudoplexaura zone, 2) a G. ventalina P. flexuosa, E. fusca zone and 3) an E. fusca, E. succinea zone. Station 9 (and 8) has three main zones: 1) a G. ventalina, P. flexuosa, M. flavida, Pseudoplexaura zone, 2) an I schrammi, M. pinnata, M. laxa zone and 3) a Lophogorgia, Ellisella zone. At Station 5, each zone clusters with its adjacent one except for Zone C which groups with the cluster of all the other zones and is not positioned in the dendrogram where expected, i.e. between B and D. This depth zone at this station has a high frequency of G. ventalina so that this species increased rather than decreased in number from the shallower zones, probably causing C to fall off of its expected position in the dendrogram. This high frequency of G. ventalina could be due to the fact that even though Station 5 is located on the eastern, protected side of the bay, it is in a more open position, close lo the north- east point and is thus more exposed than Stations 3, 7, 8, 9. 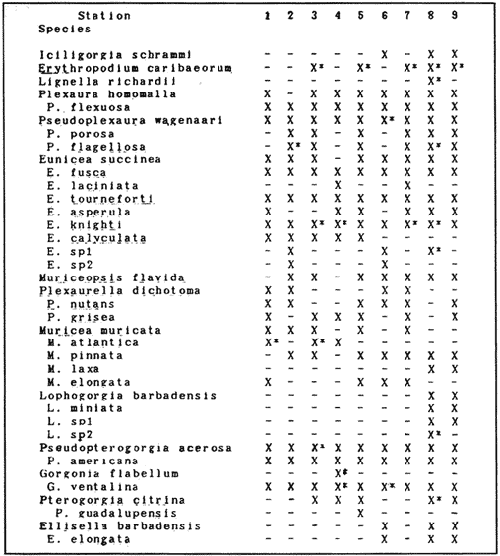 TABLE 2 SPECIES FOUND IN THE STUDY AREA. *NOT PRESENT WITHIN THE SAMPLINGT RECTANGLE BUT CLOSE BY 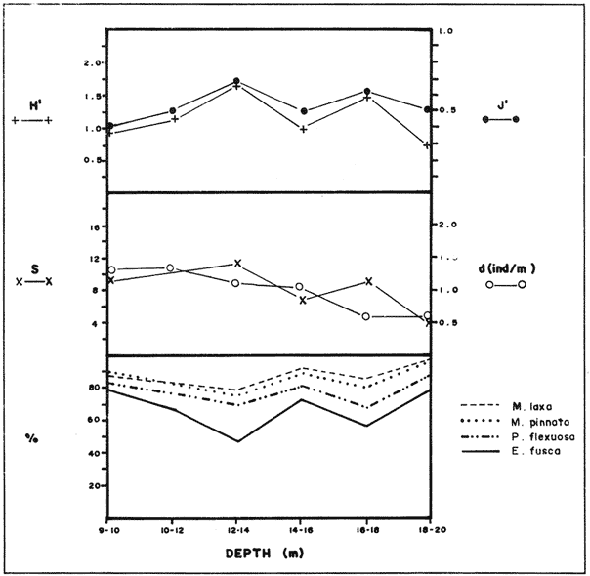 Figure 3. Data for the 6 depth zones of the sampling rectangle in Station 2. Top graph: heterogeneity (H') and equitability (J') indexes. Middle graph: species richness, (S) and density (d) of gorgonians. Bottom graph: cumulative percentage composition of most abundant species at each of the zones. A cluster analysis was also perfomed to compare whole stations with each other (Fig. 11) on the basis of species distribution and frequency. Resulting clusters are very close to what might be expected from the previous qualitative and quantitative observations of these communities. Stations 8 and 9, which are the only ones characterized by a steep slope, a hard substratum down to 45 m depth, and located on the protected sides of islets, are clustered together. Stations 3, 5 and 7, which are located adjacent to the coastline on eastern, (protected) sides of bays and have hard substrata (rocky or coralline) reaching down to 13-14 m depth are likewise clustered together. Stations 3 and 7 are more similar lo each other (CZ=0.82) than to Station 5, and this is why Station 5 clusters to them at a lower similarity level (CZ=0.53). This could reflect the fact that Station 5 is located closer lo the eastern, outer-most point of the bay and is thus somewhat more exposed than stations 3 and 7. Stations, 1 and 4, which are the only two stations located on exposed sites of bays, are clearly grouped together. The group formed by Stations 8 and 9 clusters to the group formed by 3, 7 and 5 by a 0.38 similarity level. Both of these groups contain stations positioned on protected sites of either bays or islets and are located adjacent to the coastfine. Stations 6 clusters to the big group formed by Stations 3-7-5/8-9 but by a low similarity level (0.25), and Station 2 clusters to the group formed by 1 and 4 also by a low similarity level (0.19). 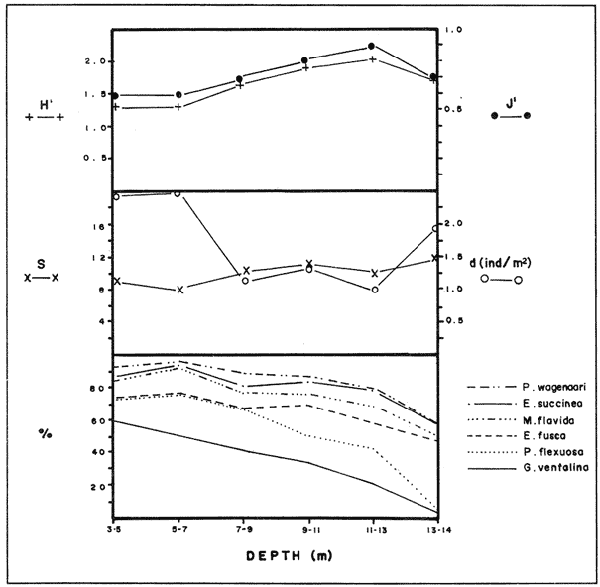 Figure 4. Data for the 6 depth zones of the sampling rectangle ín Station 3. Top graph: heterogeneity (H') and equitability (J') indexes. Middle graph: species richness (S) and density (d) of gorgonians. Bottom graph: cumulative percentage composition of most abundant species at each of the zones. 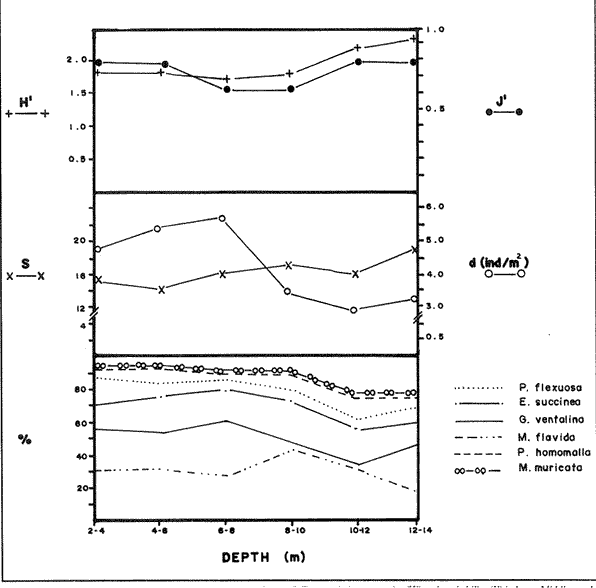 Figure 5. Data for the 6 depth zones, of the sampling rectangle in Station 5. Top graph: heterogeneity (H*) and equitability (J') indexes. Middle graph: species richness, (S) and density (d) of gorgonians. Bottom graph: cumulative percentage composition of most abundant species al each of the zones. 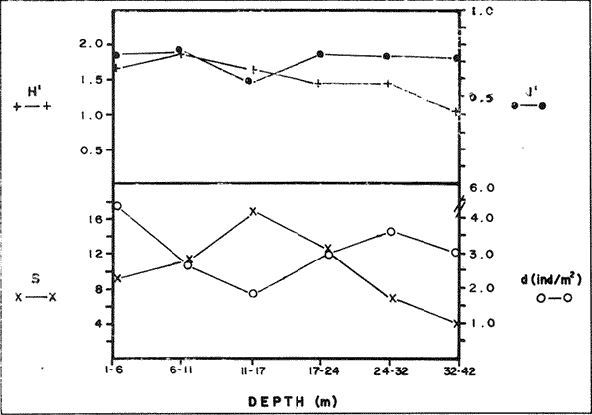 Figure 6. Data for the 6 depth zones of the sampling rectangle in Station 9. Top graph: heterogeneity (H') and equitability (J') indexes. Bottom graph: species richness (S) and density (d) of gorgonians. DISCUSSION
SPECIES COMPOSITIONIn spite of the prevailing physical environmental conditions, Gorgonacea of Santa Marta Tayrona National Park are abundant and diverse. The number of species found in this first investigation of the area (38 species) is comparable to that reported by Gonzalez-Brito (1970b) for Puerto Rico (39 species), Goldberg (1973a) for Miami (29 species), Kinzie (1973) for Jamaica (42 species), Opresko (1973) for Miami (29 species), Alcolado (1981) for Cuba (39 species), and Muzik (1982) and Lasker and Coffroth (1983) for Carrie Bow Cay, Belize (36 species). Pseudopterogorgia elisabethae, a species that is absent from TNP, is found at other Caribbean locations in fairly deep water (30-40 m) (Goldberg, 1973a; Kinzie, 1973) because it is not resistant to much water agitation. It is possible that its absence from TNP is due to its inability to withstand the agitation characteristic of most shallow waters in the area, while at greater depts it would not get the necessary amount of ligh required by its symbiotic zooxanthellae or find hard substratum neccessary for settlement and growth. Similar environmentally-related reasons could probably apply to other species such as Pterogorgia anceps, Muriceopsis petila and Briareum asbestinum that consistently appear at places with more "typical Caribbean conditions" (Jamaica, Cuba, Belize) but are absent from Tayrona National Park. Temperature could be another factor responsible for exclusion of some of these species, not so much for its relatively low values during the upwelling season (species absent from the study area are present in the area of Miami where winter water temperature is as low as 20-22°C) but because of the sudden and abrupt changes (5-6°C in 3-4 days) that occur at the onset of the upwelling period. 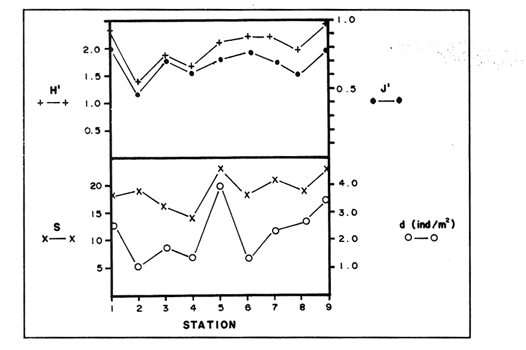 Figure 7. Data for the 9 depth zones of the sampling rectangle in Station 9. Top graph: heterogeneity (H') and equitability (J') indexes. Bottom graph: species richness. (S) and density (d) of gorgonians. 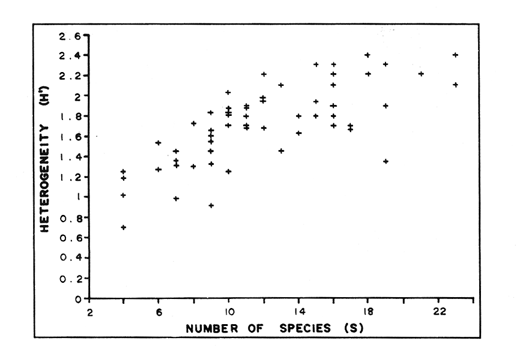 Figure 8. Relationship between the Shannon Weaver index of diversity (H') and species richness (S). Each point represents one zone in each of the nine sampling stalions, ora station pooled as a whole. 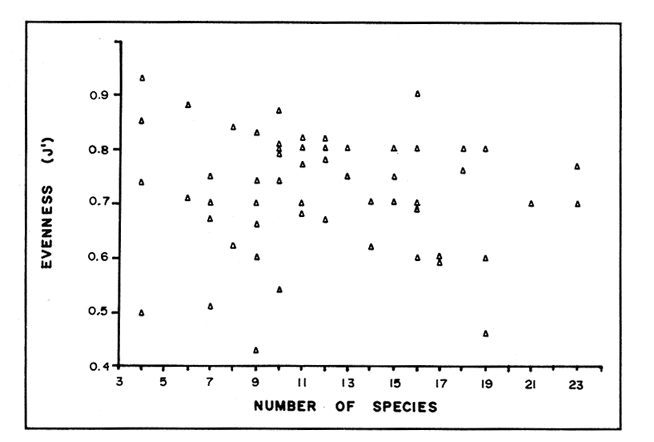 Figure 9. Relationship between the index of equitability (J') and species richness (S). Each point represents one zone in each of the nine sampling stations, or a station pooled as a whole. DIVERSITY AND EQUITABILITY OF COMMUNITIESEither a temporally unpredictable or a temporally constant but physiologically stressful environment should constrain the development of high species diversity since relatively few species would he able lo colonize the environment successfully (Preston and Preston, 1975). However, equitability in the distribution of individuals among species should be different in the two environments: in a constant but severe environment equitability should be relatively high in late successional stages. In an unpredictable environment, catastrophic disturbances can keep the ecosystem at a low state of maturity and both, species diversity and equitability would be low (Preston and Preston, 1975). Regarding heterogeneity and equitability indexes, which in my sampling stations ranged from 0.7 to 2.4 and from 0.4 to 0.9, respectively, several aspects can be discussed. Taking into account the total number of species found in the area (38), maximum possible H' would be 3.6 (H'=InS). However, one station never had more than 23 species and in this case maximum possible H' be 3.1. Highest H' encountered was 2.4 for Stations 1 and 9 as a whole and 2.3 at 12-14 m depth in Station 5 and 10 m depth in Station 1. According to Margalef (1969), H'. tends to vary from near 0 bits in immature communities or those in unpredictable or severe environments to a maximum near 5 bits (=3.5 using natural logarithms instead of log 2) in mature communities in predictable environments. Preston and Preston (1975) found H' values of 3.46 bits, (=2.39 using natural logarithms) for gorgonian communities in Puerto Rico and interpreted their value as high thus concluding that gorgonians interpreted their environment as predictable and favorable. Accordingly, my data indicate that gorgonians of Tayrona National Park are in a predictable and favorable environment since both H' and J' are relatively high at all stations (each station taken as a whole). Values of S, H'and J'are not signifícantly different among the different stations except for Station 2 that Station 2 that has significantly lower H' and J' both per zone and pooled as a whole. This could reflect the low water agitation atthis site (see position of this "Piedra Ahogada" in map: in inner and protected part of bay) and the almost constant turbidity caused by suspended sediment particles transported by currents from adjacent muddy bottoms. Barham and Davis (1968) reported that gorgonians are not found in areas where there is no water movement, but occur where there are moderate oscillations. Approximately 50% of the zooxanthellate gorgonian species so far reported from the Caribbean Sea, were found in the present investigation in a total sampled area of 5770 m². They were vertically distributed only between 0-22 m depth. Similar precentages in other Caribbean areas include wider depth distribution ranges. This indicates that in spite of the narrower vertical range, the part of the environment in Tayrona National Park that is suitable for zooxanthellate gorgonians is sufficiently heterogeneous as to provide for all these species. 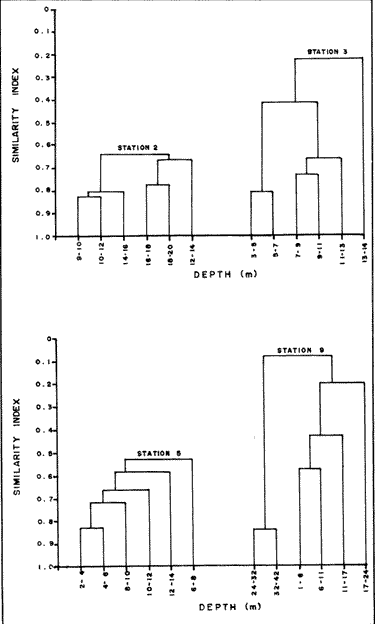 Figure 10. Dendrogram display of cluster analyses of species composition and frecuency in the 6 depth zones of Stations 2, 3, 5 and 9. 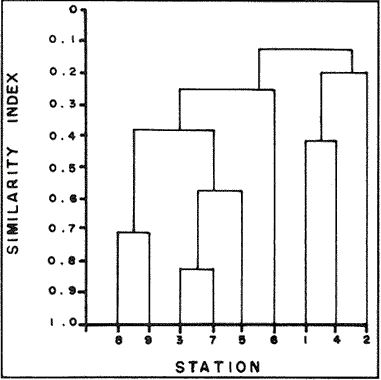 Figure 11. Dendrogram display of cluster analyses of species composition and frecuency in the 9 sampling Stations, each station considered as a whole. Three separate studies on well developed reefs have found similar patterns of seleractinian species diversity (measured by species richness S, and/or H') in relation to depth (Loya, 1972; Sheppard, 1980; Huston, 1985b). On each reef, diversity was low near the surface and increased to a maximum between 15-30 m depth, and then decreased gradually. At my study area gorgonian species richness (S) and H' generally increased from shallowest zones to depths around 13-16 m. At Stations 3, 5, and 7 these depths were also the lowest limits of gorgonian vertical distribution since there was no adequate substratum below. At Stations 8 and 9, gorgonian depth limit was 40-45 m and suitable substratum was available at these depths; highest S was measured around 15-22 m depth and below this zone S decreased gradually. Thus, gorgonians were following the same pattern of diversity (relative to depth) of scleractinians. The fact that at Stations 8 and 9, azooxanthellate species appear below the depth of maximum S, and that the number and density of zooxanthellate species decreases below this depth, could indicate that light (as the one factor directly related to depth) is the main abiotic factor structuring the diversity and zonation of the gorgonian communities provided that suitable substratum is available. Species composition and the distribution of individuals among species is different between stations and between zones of stations, and this is clearly depicted in the cluster analyses and quantitative zonation profiles. Visualin situ observations had suggested that there were three main types of gorgonian communities in the Santa Marta Tayrona National Park area: 1) communities located at 9-10 m of depth on exposed and agitated sides of bays, where Plexaurella grisea is dominant (though at Station 4 Muricea atlantica is more abundant numerically, the large sized (almost 5 times the height of M. atlantica) and abundant P. grisea dominates the visual appearance of the community); 2) communities located between 0.5 and 15-16 m of depth in eastern protected sides of bays where botton profiles have slopes of 15-30 and pronounced relief of scleractinian heads and where Gorgonia ventalina, Plexaura flexuosa, Muriceopsis flavida Eunicia succinea, and/orE. fusca are the most abundant gorgonians; and 3) communities located on protected sides of islets between 0.5 m of depth, where bottom slopes range from 20 to 55 and hard unconsolidated substratum reaches down to 45 m; these are the sites where azooxanthellate species were present and abundant. Cluster analyses (Fig. 11) corroborated the existence of these "community types". These three "community types" are clearly a result of prevailing environmental conditions, mainly agitation, substratum and light availability. AgradecimientosThis research was funded by COLCIENCIAS. I am grateful to Dr. M. Carriker for constructive comments to earlier drafts and to Dr. G. Bula-Meyer for his help and support during fieldwork. LITERATURAALCOLADO, P.M. Informe Cientifico-Técnico # 187 zonacion de los gorgonaceos someros de Cuba y de su posible uso como indicadores comparativos de tensión hidrodinamica sobre los organismos del bentos. Acad Cienc. Cuba, Inst. Oceanol.1981. 43 p. ANTONIUS A. Mitt. Inst. Colombo-Aleman Invest. Cient. Occurrence and distribution of stony corals (Anthozoa and Hydrozoa) in the vicinity of Santa Marta Colombia.1972.89-103. 6 BARHAM. E. and J. DAVIS. The Underwat. Nat. Gorgonians and water motion studies in the Gulf of California.1968.24-28.(3): 5 BAYER. F. M. Martinus Nijhoff the shallow water Octocorallia of the West Indian region.The Hague1961 373 pp. BLOOM, S.A. Mar. Ecol. Progr. Ser. Similarity indices in community studies: potential pitfalls. 1981. 125-128. 5: BOTERO, L. Doctoral Dissertation. Gorgonian octocoral communities of the Santa Marta area, Caribbean Coast of Colombia: species composition, patterns of zonation and quantitative structure. University of Delaware1987a. 136 p. BOTERO, L. Anales Inst. Inv. Mar. Punta Betin Zonacion de octocorales gorgonaceos en el área de Santa Marta y Parque Nacional Tayrona, costa Caribe Colombiana. 1987b.61-80.17: BULA-MEYER, G. An. Inst. In v. Mar. Punta Betin Algas marinas benticas indicadoras de un área afectada por surgencia frente a la costa Caribe de colombia. 1977. 45-71.9: BULA-MEYER, G. Bol. Ecotropica Un nucleo nuevo de surgencia en el Caribe colombiano detectado en correlacion con las macroalgas. 1985.3-25.12: CAIRNS, S. Sea Grant Field Guide Series # 6. Guide to the commoner shallow water gorgonians (sea whips, sea feathers and sea fans) of Florida, the Gulf of Mexico and the Caribbean Region. G. Voss (ed). Univ. Miami Sea Grant Program1977. 74 pp DINESEN, Z.D. Coral Reefs Patterns in the distribution of soft corals across the Central Great Barrier Reef. 1983.229-236.1: GOLDBERG, W.M. Bull. Mar. Sci. The ecology of the coral-octocoral communities off the Southeast Florida coast: Geomorphology, species composition and zonation. 1973a. 465-488.23: GONZÁLEZ-BRITO, P.Bol. Inst. Oceanogr. Algunos octocorales de la Isla de Margarita, Venezuela. Univ. Oriente 1970a. 79-92.(1-2): 9 GONZÁLEZ-BRITO, P. Carib. J. Sci.Una lista de los octocorales de Puerto Rico.1970b. 63-69. 10: HUSTON, M.A. Bull. Mar. Sci. Patterns, of species diversity in relation to depth at Discovery Bay, Jamaica.1985b.928-935.37: JORDAN, E. An Centro Cienc. Mar y Limnol. Univ. Nal. Auton. Mexico An analysis of gorgonian community in a reef calcareous platform on the Caribbean coast of Mexico. 1979.87-96.(1): 6 JORDAN, E. M. MERINO, O. MORENO and E. MARTIN. Proc. 4th Int. Coral Reef Symp. Community structure of coral reefs in the mexican Caribbean.1981.303-308.2: JORDAN. E. and R.S. NUGENT. An. Centro Cienc. Mar y Limnol. Univ. Nal. Auton. México Evaluación poblacional de Plexaura homomalla (Esper) en la costa noroeste de la Península de Yucatán (Octocorallia). 1978.189-200.(1): 5 KINZIE, R.A. III. Ph. D dissertation The ecology of the gorgonians (Cnidaria, Octocorallia) of Discovery Bay, Jamaica. Yale University.1970. KINZIE, R.A. Bull. Mar. Sci. The zonation of West Indian gorgonians. 1973. 93-155.23: LASKER, H.R. and M.A. COFFROTHMar. Ecol. Progr. Ser., Octocoral distributions at Carrie Bow Cay, Belize. 1983. 21-28.13: LOYA, Y. Mar. Biol. Community structure and species diversity of hermatypic Corals at Eilat, Red Sea. 1972. 100-123.13: MARGALEF, R. In: Diversity and stability in ecological systems Brookhaven Symp. on Biol. Diversity and stability: a practical proposal and model Of interdependence. Brookhaven National Laboratory 1969. p. 25-37. MUZIK K. Smithson. Contrib. to the Mar. Sci., # 12 Octocorallia (Cnidaria) from Carrie Bow Cay, Belize. In: The Atlantic Barrier Reef Ecosystem at Carrie Bow Cay, Belize, I. Structure and communities. K. Rutzler and I.G. Macintyre (eds). Smithsonian Institution PressWashington1982. p. 303-310. OPRESKO, D.M. Bull. Mar. Sci. Abundance and distribution of shallow water gorgonians in the area of Miami Florida. 1973. 535-558.23: PRESTON, E.M. and J.L. PRESTON.Bull. Mar. Sci. Ecological structure in a West Indian gorgonian fauna. 1975. 248-258.25: RAMÍREZ, G. An. Inst. Inv. Mar. Punta Betin Caracteristicas fisicoquímicas de la Bahia de Santa Marta1983.111-122.13: (agosto 1980-julio 1981). REES, J.T. Carib. J. Sci. Shallow-water octocorals of Puerto Rico: species account And corresponding depth records. 1973. 57-58.(1-2): 13 SALZWEDEL, H. and K. MULLER. An. Inst. Inv. Mar. Punta Betin A summary of metheorological data from the bay of Santa Marta. Colombian Caribbean.1983. 67-84 13: SCHEER, G. Application of phytosociologic methods In: Coral Reefs: research methods. D.R. Stoddart and R.E. Johannes (eds). UNESCO 1978. p. 175-196. SHEPPARD, C.R.C. Mar. Ecol. Progr. Ser. Coral cover, zonation and diversity on reef slopes of Chagos Atoll and population structures of the major species. 1980.193-205.2: TURSCH, B. and A. TURSCH.Mar. Biol. The soft coral community on a sheltered reef quadrat at Laing Island (Papua, New Guinea).1982.321-332.68:
|

