|
AEROBIC DIGESTION IN ORGANIC
WASTE TREATMENT FOR THE MICROALGAE GROWTH
Trabajo recibido el 20 de enero
de 1987 y aceptado para su publicación el 16 de diciembre de
1987.
J. Paniagua-Michel
F. Bückle-Ramírez
A. Chagoya-Guzmán
Division of Oceanology, Centro de Investigación Científica y de
Educación Superior de Ensenada, Espinoza 843, Ensenada, Baja California,
México.
En biorreactores
discontinuos se investigó la carga orgánica y el tiempo
óptimo requeridos por las excretas de vaca y de gallina y el alga
[Macrocystis pyrifera] Agard, para obtener los nutrientes necesarios en el
cultivo de microalgas marinas. Con la técnica de superficies de
respuesta se calculó para cada biorreactor la carga orgánica que
gcnera la mejor nitrificación en función de la reducción
de los sólidos volátiles suspendidos (RSSV), la razón de
la demanda biológica de oxígeno (BOD) y la demanda química
de oxígeno (COD). Estimamos que para 11 litros de agua dulce se
necesitan 550, 420 y 660 g de excretas de gallina, de vaca y (M. pyrifera)
respectivamente. Al preparar el medio de cultivo se eligieron los extractos
biodigeridos correspondientes a los 25; 10 y 15 días de tratamiento de
las excretas de gallina, vaca y macroalgas respectivamente, formando una mezcla
en la cual se mantuvo la razón nitrógeno-fósforo necesaria
para el crecimiento de las mícroalgas. Los cstractos obtenidos en los
biorreactores sirvieron como fuente de nutrientes y sustancias promotores del
crecimiento (GPS) para el cultivo de (Pavlova lutheri) Green. La densidad de
las microalgas cultivadas fué similar a aquellas alcanzadas con el medio
control al 95% de intervalo de confianza.
Organic load and
the best treatment time measurements were made for cow and chicken manure and
the seaweedMacrocystis pyrifera Agard. In abtch-fed
bioreactors, to obtain nutrients for microalgae growth. Both the organic load
for optimum nitrification in function of the reduction of suspended volatile
solids (RSSV) and the ratio of biological oxygen demand (BOD) to chemical
oxygen demand (COD) were calculated for each bioreactor by the surface response
technique. We estimate that 550, 420, and 660 grams of chicken manure, cow
manure andM. pyrifera respectively are required per 11
liters of tapwater. Growth medium was prepared choosing the biodigested
extracts of 25, 10 and 15 days of treatment of manures of chicken, cow, and the
seaweed, respectively, in mixtures where the ratio nitrogen to phosphorus
required for microalgal growth was mantained. The biodigested extracts obtained
were used as a source of nutrients and growth promoting substances (GPS) for
the culture ofPavlova lutheri Green. The microalgal
densities were similar to those attained in the control medium at 95% of
confidence interval.
The world need for proteins has focussed the attention of scientist in developing countries in the search of new technologies to obtain food from the wastes. The possibility of increasing food sources by artificial cultivation of shellfish in a hatchery environment has awakened the interest in the biomass production of microalgae (Ukeles, 1980). Microalgal production in shellfish hatcheries in developing countries need to be directed towards the design of low cost growth media. Although several methods exist for the treatment of organic waste, one reliable technique for the recovery of nutrients from animal and vegetable wastes required for microalgal growth is the aerobic digestion. This technique consist either in the treatment process whereby organic compounds are oxidized to estable organic and inorganic end products (Brody, 1979), or the destruction of degradable organic sludges by aerobic biological mechanisms (Adams, et al., 1974). In the start up, design and operation of aerobic digestion systems (bioreactors = biodigesters), the following steps need be taken into account: estimating the quantity of sludge (organic load) from the digestion tank; establishing digester's volume; determining oxygen requirements; and selecting aeration equipment for oxygen transfer and mixing (Brody, 1979). Eckenfelder (1956) was among others that investigated nutrient changes during digestion. Others authors have used aerobic digestion in wastewater treatment (Loehr, 1965; Bishop and Farmer, 1978; Koers and Mavinic, 1977; Lynam. et al., 1967 and Pajak and Loehr, 1975). Only a few have applied this technique to microalgae cultivation under controlled conditions (GranadosMachuca and Bückle-Ramírez, 1984; Paniagua-Michel, 1984; Paniagua-Michel and Buckle-Ramírez, 1985; PaniaguaMichel et al., 1987). In this study, we measured the optimal organic load and the best treatment time of cow and chicken manure and the seaweed M. pyrifera in batch-feed bioreactors to obtain nutrients for the culture of the microralgae P. lutheriunder controlled conditions. MATERIAL AND METHODSEach waste was processed separately in aerobic batch-feed polypropylene biodigesters (Fig. 1) of cylindrical shape, 18 1 in volume, 43 cm in height and 28 cm in diameter. Inside each biodigester, an 11 cm thick and 551,21 cm² area bed of gravel, 2-5 mm in diameter, was laid at the bottom (Fig.1- 1), and a perforated bucket, held in suspension by a hollow stand, was instaled (Fig. 1-11). The raw products were introduced for lixiviation, elutriation and transformation of their carbonaceous and nitrogenous sources. The container's sever lead to a duct connected to a round cuvette housing an immertion heater (Fig. 1-3). From the cuvette, a fitting was coupled with a liquor sampler stopcock (Fig. 1-4) and an air injection valve (Fig.1-5) for supplying oxygen to the nitrification processes and for mixing and rising the liquor to the top of the biodigester. The ascending duct, at its terminal portion, had a tee union with a thermometer (Fig.1-6) to read the treatment temperatures and an extension to pour back the liquor directly over the perforated bucket by means of a rudimentary sprinkling system (Fig.1-10); all parts were of PVC. The biodigester was covered to avoid waters and scum losses. The biodigester's lid had a duct for venting gases (Fig.1-8) with an intermediate scum fractionator to block up the scum generated during the process (Fig. 1-7). The liquified scum reentered the unit through a security spout (Fig.1-9). The required values of organic load for optimal nitrification and maximum recovery of nutrients were obtained in a first experiment. Each digester was fed empirically with 1 kg of its corresponding resource placed in the perforated bucket (Fig.1-11), and 11 l of tapwater were added. Biodigesters 1 and 2 were fed with fresh manures from chicken and cow respectively, collected from local dayries. Biodigester 3 was loaded with M. pyrifera from a kelpbed; stipes and blades were grinded. The retention times of the liquors in digesters were estimated acording to Vavilin (1982): 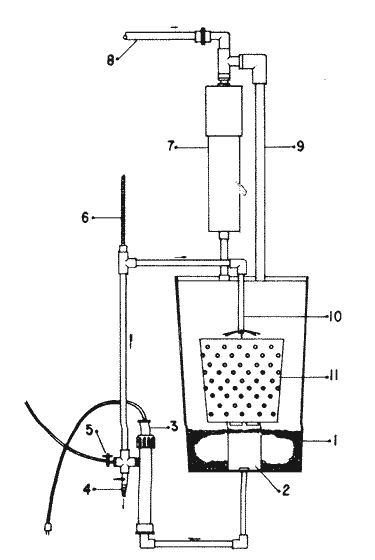 Figure 1. Laboratory Digester System. 1) Gravel bed; 2) Bucket support; 3) Heater, 4) Sampling stopcock; 5) Air injection valve; 6) Thermometer 7) Scum fractionator, 8) Gas venting bucket; 9) Security spout; 10) Sprinkler, 11) Perrorated bucket. Arrows indicate liquid flow. T=DA/q Where D is the depth of the filter bed, A its cross sectional area and q the specific hydraulic loading. The mixed liquor temperature was set at 28 + - 1°C. Mixed liquors were sampled every other day until the tenth day of treatment, and thereafter, every five days during 30 days. The liquor samples were analyzed with the following analytical methods: dissolved oxygen measured by the modified Winkler method; pH determinated with a Hach pH meter model DR-EL/4 (1980); biomechemical oxygen demand (using the azide modification of the iodimetric method); chemical oxygen demand; volatile suspended solids; nitrate concentration (measured with a 901 Orion Ion Analyser) and flow rate. All analysis were performed as described in A.P.H.A. (1980). The volatile suspended solids reduction (RSSV) was measured as described by Bishop and Farmer (1978): RSSV = 1 - St/So Where St is defined as the concentration of degradable volatile suspended solids remaining at any time, and So is the initial concentration of degradable volatile suspended solids. Descriptions of the effects of BOD/COD and RSSV on nitratification efficiency (percent of nitratification) were carried out by response surface analysis (Alderdice, 1972): N = C¹ + C2X + C3Y + C4X2 + C5XY + C6Y2 Where N = percent response (total nitratification); X = RSSV, Y = BOD/COD, mg/l. The raw manures and seaweed were analyzed for their total Kjeldhal nitrogen (TKN) by the macroKjeldhal procedure; lipids, ash and moisture were determined as recomended by AOAC (1980). With the data generated, the proper organic load was calculated and a second experiment, was carried out feeding the biodigesters with the organic load previously estimated. Samples were withdrawn every five days during the experimental period (30 days), pH and dissolved oxygen levels were measured similarly as in the first experiment. Ammonia, nitrate, phosphate, silicate, iron, cooper and manganese concentrations were analyzed according to the procedures described in the Hach Manual (1980). Also 1200 nil of each biodigesteds extracts were stored frozen for later growth medium elaboration, after lowering the pH with hydrochloric acid 1N to 5 and processing them individually in an autoclave at 121° C and 15 lb/inch². The biodigesteds extracts obtained from the experimental period were analysed, and those with the highest nutrient concentration of each biodigested waste (M. pyrifera; cow and chicken manure) were selected and processed to achive a mixture with a nitrogen to phosphorus ratio (N:P) of 15 (in weight). The frozen stored extracts (1200 ml) were then thawed for the experimental growth medium elaboration. The experimental medium was designed according to Paniagua-Michel and Granados-Machuca (1981) keeping the proportions of 40:20:10 ml of the previously selected extracts from M. pyrifera, cow and chicken manure respectively per 1000 ml of seawater. To test the suitability of the biodigested extracts as a source of nutrients, the Pavlova luthery was grown in experimental and control media (Mathiessen and Toner, 1966). Algal cultures were kindly provided by Dr. William H. Thomas from the National Marine Fisheries Service at La Jolla, California and cultured in acrylic chambers under unialgal conditions, al 21 + - 1°C. Ilumination was provided by coolwhite fluorescent lamps (2152 lux), and aeration with a stream of filtered air. Each set of cultures comprissed nine replicate batch cultures of each medium (experimental and control), inoculated with an equal number of Pavlova lutheri cells from cultures that had reached a logarithmic phase. The growth of the cultures was measured in cell numbers by direct counting of cells inmovilized and stained with Utermöhl solution in a Fush Rosenthal chamber, 0.2 mm deep. The logarithmic growth phase of each algal assay was measured in a semilog (base 10) plot of algal density versus time. The equation defining this growth phase was done by least squares linear regression analysis. In all the replicate assays, daily division rate, K, (formulated by Guillard, 1975) measurements were determined in both culture media (experimental and control). RESULTS AND DISCUSSIONIn the first experiment, the biodigested extracts did not show a significative nitrate formation in spite of controlled temperature, pH, and dissolved oxygen levels in the nitrification ranges (Table 1) (Wild, et al., 1971; Bishop and Farmer, 1978; Prakasam and Loehr, 1972). Probably the organic load fed to the digester was high in BOD levels which increased the levels of ammonia and heterotrophic bacteria that supress the autotrophic bacteria required for nitrification. These factors could have restrained the formation of nitrates by reduction of the oxidation rate of nirogenous matter. 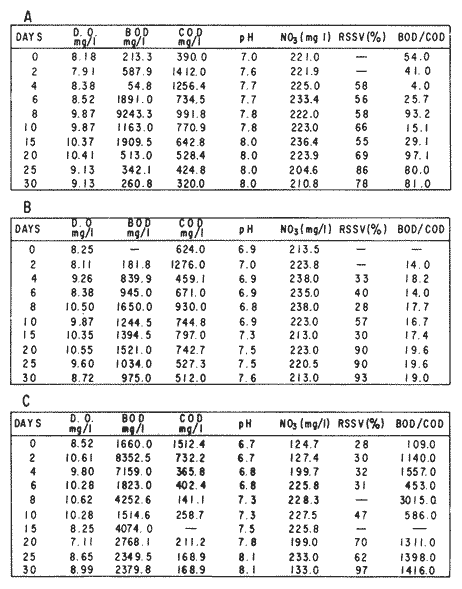 TABLE, 1 MEASURED VARIABLES OF ORGANIC LOAD IN THE THREE BIODIGESTERS. (A), CHICKEN; (B), COW AND (C), M. pyrifera It is generally assumed that when the COD levels are lower than the BOD, as was the case in this study (Figs. 2, 3 and 4), residues could easily be degradated. Hogg and Ganczarczyk (1973) have reported that digestion of activated sludge reduce the COD levels by 40 %. In this study, the wastes from the three biodigested showed the major reduction of suspended volatile solids (RSSV) after 15 days of treatment (Fig.2, 3 and 4). Treatment temperature of 28 + - 1°C encouraged the RSW rate to maximum values of 86,93 and 97% of the extracts in biodigesters 1, 2 and 3 respectively (Table 1). Jaworsky (1960) (mentioned by Lawton and Norman, 1964) reports that an increase in digestion temperatures from 15 to 35°C stimulates the RSSV rate. Temperatures below 20°C were significantly retardant to digestion (Randallet al., 1975), since at low temperatures microorganisms require more time to metabolize organics from wastes. 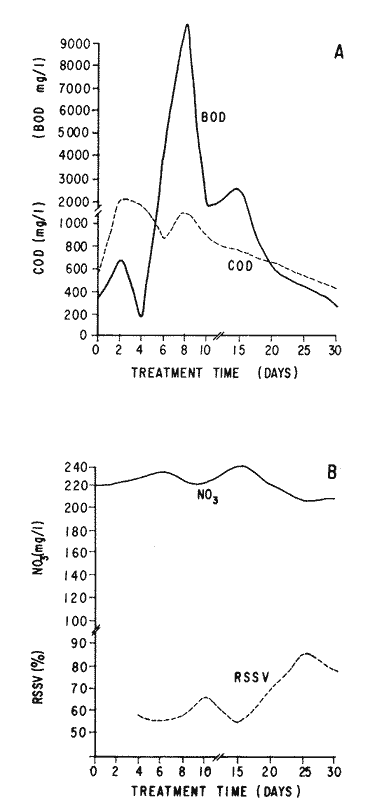 Figure 2. COD, BOD ( A ), NO3 and RSSV ( B ) measurements in the chicken manure extracts. 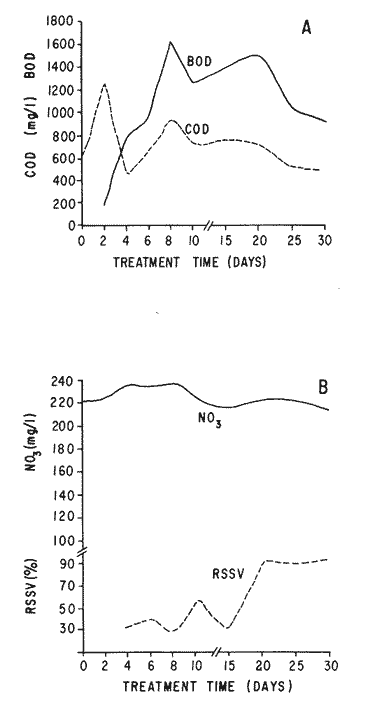 Figure 3. COD, BOD ( A ), NO3 and RSSV ( B ) in the cow manure extracts. Due to the poor nitrification atteined in this first experiment, we calculate the organic load for optimum nitrification in function of RSSV and the loading factor BOD/COD. This was achived by the surface response technique. Hence, for biodigester 1, the optimum nitrification (99%) was at an organic load factor of 50% and a RSSV of 55% (Fig. SA), which corresponds to 550 grams (in 11 l of tapwater) of chicken manure. 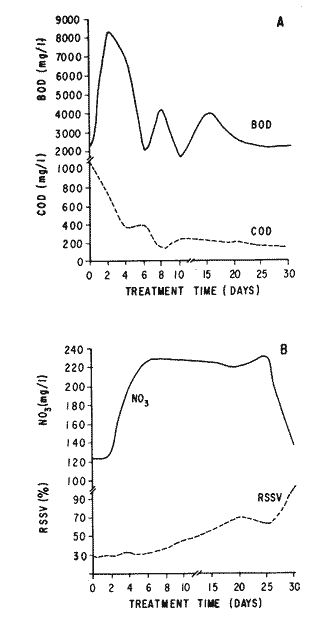 Figure 4. COD, BOD ( A ), NO3 and RSSV ( B ) in the M. pyrifera extracts. N = 70,39 + 1,22 (RSSV) - 9,13 (BOD/COD) + 0,013 (RSSV)² + 44,75 (RSSV) (BOD/COD) + 18,69 (BOD/COD)² This explain a 92.38% of the nitrates variability in function of the RSSV and the BOD/COD) + 18,69 (BOD/COD)2 This explain a 9238% of the nitrates variability in function of the RSSV and the BOD/COD. In digester 2, 42 % of RSSV was necessary for optimal nitratification (100%) whit a loading factor (BOD/COD) of 19,00 (Fig. 5B), which corresponds to 420 grams (in 111 of tapwater) of cow manure. The second order polynomial explains a 89,91 % of the nitrates variability. The equation defining this is: N = 103,4 + 1,47 (RSSV) - 16,11 (BOD/COD) + 0.96 (RSSV)² 0,88 (RSSV) (BOD/COD) + 6,18(BOD/COD)² The optimal nitraritication response (100%) in the biodigester 3 was achived at a loading factor of 11.60 and 66 % of RSSV (Fig. 5C), corresponding to 660 grams (in 11 l of tapwater) of M. pyrifera. The polynomial model defining the 71% of nitrates variability is as follows: N = 35,96 + 4,49 (RSSV) + 0,090 (BOD/COD) - 0,27(RSSV)² 0,08(RSSV) (BOD/COD) + 0,00010 (BOD/COD)² In the three biodigesters, contours of best nitrification tended toward high levels of the BOD/COD ratio. This behavior is an index of the good perfomance of the system. The BOD/COD ratio increases in the course of biological treatment since the range of the remaining compounds tends towards those that are oxidized less readily (Vavilin, 1982). This ratio also provides an index of the amenability of the wastes to biological treatment (Stones, 1981). The maximum nitrification toward low RSSV values in biodigesters 1 and 2 (Figs. 2 and 3) and high in 3 (Fig.4) could be due to the high contents of organic nitrogen in the cow and chicken manures (Table 2), and the fact that high reduction rate for the release of nitrates is not needed since manures are in the form of a composite paste.M. pyrifera has a high cellulosic contents that requires higher RSSV for a major nitrification. In the second experiment, the nitrification rate increased (Fig.6). Maxima nitrate concentrations in bioreactor 1 (660 mg/l) were almost three times higher than those in the first experiment (236,4 mg/lNO3); almost twice as great in bioreactor 2 (Fig.7) and slightly above in bioreactor 3 (Fig. 8). In general, chicken manure has the highest nitrate concentration due to its high contents of organic nitrogen (Table 2), and the balanced feed that the chicken receive. Cow manure andM. pyrifera contain cellulosic and hemicellulosic compounds of difficult descomposition (Lodha, 1974). Although nitrogen in the chicken manure is in the form of proteins and uric acid (Martin and Loehr, 1976) its nitrification process is shorter than the one required for cellulosic decomposition. 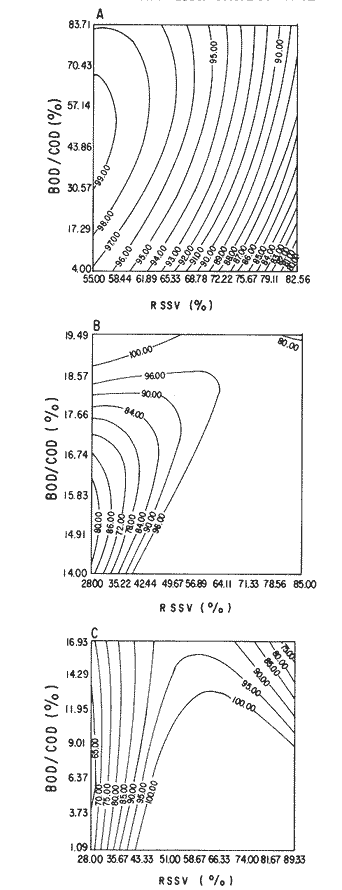 Figure 5. Nitrate (%) obtained in function of the ratio BOD/COD and RSSV in chicken manure (A), cow manure (B) and M. pyrifera (C) extracts. 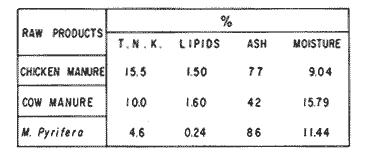 TABLE 2 CHEMICAL ANALYSIS OF THE RAW PRODUCTS The temperature in all biodigesters was in the optimal range for nitrification (28 + - 1°C). The pH registered in the three biodigesters during maximum nitrification was neutral and slight1y alkaline (7,3 - 8,2); this agrees with Wild and McMahon (1971), who reported maximum nitrification at pH 7,0 and 8,4. Maximum phosphate concentration was obtained in chicken manure at 25 days of treatment (Fig.6). Cooke (1983) points out that this manure needs long processing times to release significative amounts of nitrates. In the cow manure and M. pyrifera both maxima were obtained at 10 and 15 days of treatment respectively. Probably in rumiants an important previous breakdown of the manure is possible due to their enlarged forestomach or rumen, which serves as a habitat for anaerobic microorganisms capable of fermenting the cellulosic fíber in the feedstuffs, and thus reducing the phosphate release time when aerobically digested. In M. pyrifera, the previous processing (chopped and blended) partially reduces the release time, since the leachate formed enhances, in part, the digestion. Newell 1972, mentioned by Fenchel (1973), reported that leaves of maerophytes undergo the highest decomposition when grinded, since the surface area for bacterial action is increased. Decomposition in marine macrophytes undergoes two types of breakdown: a mechanical one and a biochemical one, both applied in this work. Bishop and Farmer (1978) attributed higher phosphates removel to "luxury biological uptake". This theory considers that a high dissolved oxygen concentration enhances phosphate uptake, which is what could have happened in our digesters. In the three units, the oxygen levels at maximum phosphate concentration were above 7,3 mg/l, which is higher than the amount required for optimal digestion. Silicates and manganese concentrations were higher in M. pyrifera than in both manures (Figs. 6, 7 and S), since seaweed absorbs these elements from surrounding waters, supporting previous findings that nutrients in the wastes depend on the origin. 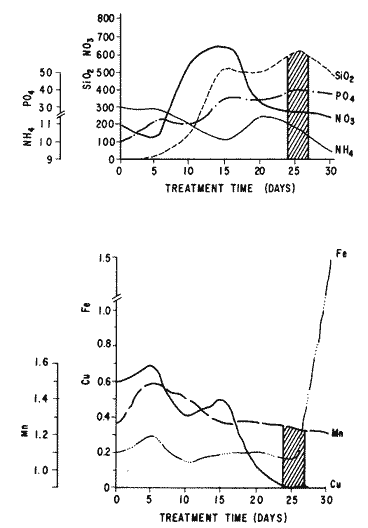 Figure 6. Nutrients (A) and trace elements (B) (mg/l) in the chicken manure extracts. The biodigests used to prepare the experimental medium is indicated by a striate bar. Iron behaved differently in the three digested extracts. In chicken manure (Fig.6) the iron concentration increased at the end of process (25 days). Teusher and Adler (1982), report that iron compounds are very soluble in the presence of ammonia ions. This agrees with our results, since we found higher iron and ammonia concentrations in the chicken manure. The experimental growth medium was made selecting the biodigested extracts with the highest concentrations of nutrients (striaded bars in Figs. 6, 7 and 8), which mixture maintained a nitrogen to phosphorus ratio (N/P) of 15 (required for phytoplankton growth in conventional culturing media). Since similar growth and division rate ofP. lutheri (Figs. 9 and 10) were attained both in the control and experimental growth media, it can be concluded that the digestion performed was efficient for the recovery of nutrients for algal growth. We also assume that the wide variety of nutrients and substances such as humic compounds, vitamins and growth promoting substances (auxins and gibberlins), elutriated and tranformed from M. pyrifera (Stephenson, 1968; Voropayev and Chechin, 1983) and the manures (Stumm, 1972), enhances the microalgal growth in the experimental growth medium. 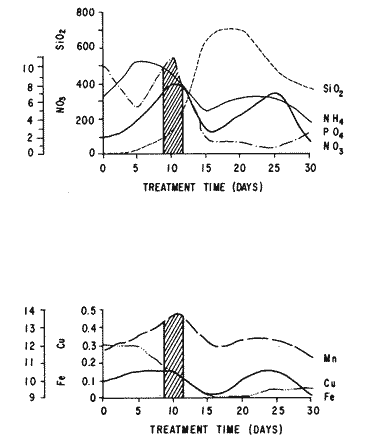 Figure 7. Nutrients (mg/l) (A) and trace elements (B) in the cow manure extracts. The biodigests used to prepare the experimental medium are indicated by a striate bar. AgradecimientosWe wish to thanks M. Sc. Claudia Farfán for her helpful descriptions of material and methods and Mrs. Aracely Granados de Mendez for the critical review of the manuscript. 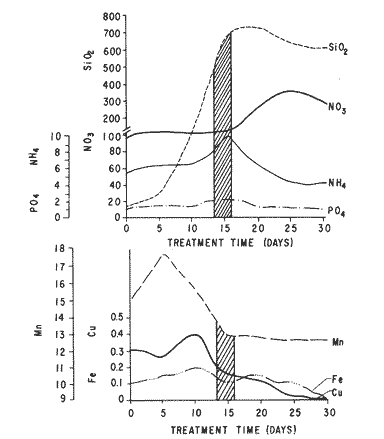 Figure 8. Nutrients (mg/l) (A) and trace elements (B) in the M. pyrifera extracts. The biodigests used to prepare the experimental medium are indicated by a striate bar. 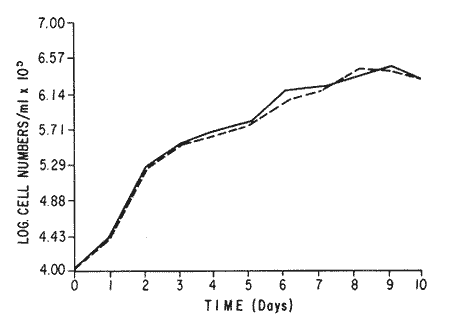 Figure 9. Growth curve of P. lutheri in the experimental (--) and the control (-) growth media. 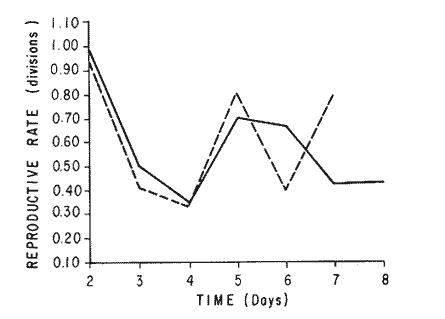 Figure 10. Reproductive rate in the experimental (--) and the control (-) growth media. LITERATURAADAMS, C.E., W. ECKENFELDER and R.T. STEIN Water Res.Modifications to aerobic digesters design.1974.213-218. 8 ALDERDICE, D.F. Marine Ecology. Factors combinations. In: O. Kinne, ed. Wiley-InterscienceLondon.1972. AOAC Official Methods of Analysis of the Association of Official Analytical Chemists W. Horwitz, ed. AOAC13th ed.Washington, D.C. 1980. APHA Standard Methods for the Examination of Water and Wastewater. APHA15th edNew York.1980. BISHOP, P.L. and M., FARMER J. Environ. Eng. Div. EEFate of Nutrients during Aerobic Digestions.1978. 967-979. 5: BRODY, O. J. Environ. Eng. Div. EE Effect of design on economy of aerobic digestion.1979.383-400. 2 COOKE, G.M. FertilizaciónCECSAMéxico. 1983. ECKENFELDER, W. Sewage and Ind. Wastes.Studies on the oxidation kinetics of biological sludges.1956.983-995. 28: FENCHEL, T. Seagrass Ecosystem.Aspects of the decomposition of seagrasses, pp. 123-143. In: McRoy and C. Heifferich, eds. Marcel Dekker Inc.New York.1973. 314 pp. GRANADOS-MACHUCA, C. and F. BÜCKLE-RAMÍREZ An. Invest. Cienc. del Mar y Linmol. Cultivo de las microalgas Monochrysis lutheri y Skeletonema costatum con nutrientes producidos por estiércoles digeridos. Univ. Nal. Autón. México. 1984. 241-256.11 GUILLARD, R.R. Culture of marine Invertebrates Animals Culture of phytoplankton for feeding marine invertebrates. In: M.L. Smith and M.H. Chanley, eds. Plenum Press New York.1975. HACH MANUAL DR - EL/4 Methods Hach Company.1980. HOGG, K-S. and J. GANCZARCZYK Water Pollut. Res. in Can.A performance study of aerobic sludge digestion.1973.26-35. 8 KOERS, D.A. and D.S. MAVINIC J Water Pollut. Cont. Fed.Aerobic digestion of waste activated sludge at low tem. peratures. 1977.460-468. 49: LAWTON, G.W. and J.D. NORMAN J. Water Pollut. Cont. Fed Sludge digestion studies.1964.201-495. 36: LODHA, R. Biology of plant litter. Decomposition of digested litter. In: C.H. Dickinson and G.J. Pugh. eds. Academic PressLondon.1974. LOHER, R. Water and Sewage Works. Aerobic digestion: fáctors affecting design.1965.169-180. 112: LYNAM B., G., MCDONNELL and M. KRUP J. Water Pollut. Cont. Fed Start up and operation of two new high rate digestion systems. 1967.518-535. 39 MARTlN, J.H. and R.C. LOHER Demostration of aereation systems for poultry wastes. EPA1976.600/2-76. MATHIESSEN, G.C. and R.C. TONER Mar. Res. Fund Inc. Possible methods of improving the shellfish industry of Martha's Vineyard. MassachussetsEdgartown, M.A.1966. 138 pp. PAJAK, A.P. and R.C. LOEHR Water Res. Treatment of poultry manure wastewater using a rotating biological contactor.1975.399-406. 10: PANIAGUA-MICHEL, J. Tesis de MaestríaCultivo de fitoplancton marino bajo condiciones controladas en un medio elaborado con productos naturales biodigeridos.CICESEEnsenada, Baja CaliforniaMéxico.1984. PANIAGUA-MICHEL, J. y F. BÜCKLE -RAMÍREZAn Inst. Cienc. del Mary Limnol. Cultivo en condiciones controladas de Monochrysis lutheri y Skeletonema costatum con extractos de macrofitas marinas. Univ. Nal. Autón. México1985.59-70.12 (1): PANIAGUA-MICHEL, J. y C. GRANADOS-MACHUCA Tesis profesional para obtener el título de OceanólogoObtención de dos mcdios económicos para el cultivo de fitoptancton bajo condiciones controladas.UABCEnsenada, Baja CaliforniaMéxico.1981. PANIAGUA-MICHEL, J., B.C. FARFÁN and F. BÜCKLE-RAMÍREZ AquacultureCulture of Marine Microalgae with Natural Biodigested Recources. 1987.249-256. 64 PRAKASAM, T.B. and R.C. LOEHR Water Res.Microbial nitrification and denitrification in concentrated wastes. 1972.859-869. 6: RANDALL, C.W., J.B. RICHARDS and P.H. KING J. Envirom. Eng. Div., EE Temperature effects on aerobic digestion kinetics. 1975. 795-811. 5 RANDALL C.W., M. SAUNDERS and P.H. KING Proc. of 18th South Water Res. Biological and chemical changes in activated sludge during aerobic digestion.1969.155-174. STEPHENSON, N.A. Conservation gardening and farming series Seaweed agriculture and horticulture. 1974. pp. 81-240. STONES, T. Water Pollut. Control. A comparison of the carbonaceous oxygen demands of a domestic and industrial sewage.1981.659-662. 53: STUMM, W. Gewässerschutz. Wasser. Abwasser. Die Rolle der Komplexbildung in natürlichen gewässern und aufällige beziehungen zur eutrophierung.1972.57-88.8 TEUSHER, H. y R. ADLEREl suelo y su fertilidad.CECSA.México.1982. UCKELES, R. Algae Biomass. American experience in the mass culture of micro-algae for feeding larvae of the american oyster Crassosttrea virginica. In: G.H. Shelef and CJ. Soeder, eds. Elsevier Biomedical Press New York.1980. VAVILIN, V.A. Ecol. Modell.Generalized model of a aerobic biological treatment. 1982.157-173. 17 VOROPAYEV, V.M. and A.P. CHECHIN Hidrobiol. J The growth of marine microalgae in cultures in the presence of gibberelins. 1983.57-59.17 (3) WILD, H.E., C.N. SAWYER and T. McMAHON J. Water Pollut. Cont. Fed. Factors affecting nitrification kinetics. 1971.1845-1853.43 (9):
|

