|
***ELECTROPHORETIC PATTERNS VARIATION IN TWO OYSTER
POPULATIONS OF Crassostrea corteziensis FROM THE MEXICAN COAST
Trabajo
recibido el 19 de mayo de 1986 y aceptado para su publicación el 26 de
enero de 1987
Faustino Rodriguez-Romero
Carlos García Saez
Alfredo Laguarda Figueras
Universidad Nacional Autónoma de
México, Instituto de Ciencias del Mar y Limnología.
Contribución 424 del Instituto de Ciencias del Mar y
Límnología, UNAM.
En todas las costas mexicanas han sido reportadas 16
especies de ostiones pertenecientes a los géneros
Ostrea y Crassostrea, en este
último se encuentran las tres especies de interes comercial que se
explotan en Mexico. Crassostrea corteziensis constituye un
recurso ostricola importante en los estados de Nayarit, Sinaloa y Sonora, en
donde se ha producido la aclimatación natural de algunas poblaciones a
ambientes caracterizados por elevada temperatura y salinidad. En este trabajo
se analizan las diferencias entre los patrones electroforéticos de dos
poblaciones procedentes del ambiente salobre e hipersalino como una posible
respuesta de la expresión genética adaptativa. " población
de San Blas presentó 17 bandas proteicas mientras que la de Teacapan
sólo presentó 14. El análisis estadístico
sugirió que ambas poblaciones tuvieron el mismo orígen.
Along the mexican coastline 16 species of oysters
belonging to Ostrea and Crassotrea genera
haye been reported, at present the thrce most important commercial species
exploted belong to Crassostraea genus. Crassostrea
corteziensis has an important potential as commercial oyster resource
in the states of Nayarit, Sinaloa and Sonora, where these organisms grow up by
natural acclimatation, even in some adverse environments characterized by high
temperature and salinity. In this work it is analyzed the differences between
the electrophoretic patterns of two oyster populations from those brackish and
hipersaline environments, in order to follow some of the possible variations of
the adaptive genetic expression. The San Blas population has shown 17
Electrophoretic bands while the Teacapan is 14. The statistic best suggested
that both populations had the same origin.
Ecological variation acts upon shape, size, color and texture of the oysters of the Crassistrea genus, even now this is the main obstacle to effect: taxonomic and systematic descriptions and studies basically supported on external morphology, (Thomson, 1954; Stenzel, 1971 and Stuardo y Martínez, 1975) because factors like substrates, salinity, current velocities, wave activity and depth are causes of diversity on shape and texture of the oyster conchs. Consequently other criteria have been attempted. One of them is the use of some anatomical characters lo discern among the Crassostrea, Ostrea and Pygnodonta genera. However at interspeciric level, Anatomy is not usefull lo discriminate oysters. On the other hand, cytogenetic studies focused on cytotaxonomy of oysters although their scarcity has given valuable criteria for karyotypic tpification on those species presently studied (Ahmed and Sparks, 1967; Ahmed, 1973; Longwell et al., 1967; Menzel, 1968; Rodríguez Romero et al., 1978, 1979a, 197%) do not give enought information for an easy discrimination of species because the stability of morphology and chromosomic number of chromosomes in their karyotypes. Refering to biochemistry-genetic studies, Hillman (1964) worked on aminoacids chromatographic patterns, and protein variability has been recently studied by means of electrophoretic techniques (Fugino and Nagayama, 1977; Johnson et al., 1972; More et al., 1966, 1971a, 1971b, Torigoe, 1978; Wilkins and Mathers, 1973; Buroker, 1979). OBJECTIVEIn order to search the Crassostrea taxonomic, systematic, phylogeny and evolutionary subjets it has brought to the practice the biochemistry genetics criteria whitin the oysters populations dilemma in the mexican coast. The objective is to discern similitudes and differences at electromorphos level between two oyster populations of Crassostrea corteziensis which were under different environmental conditions. MATERIALS AND METHODS
OYSTERSCrassostrea corteziensis oysters were collected in june 1982, in two localities on the mexican pacific coast. The first ones: Estero del Pozo, San Blas, Nayarit, where wild populations are exposed to variable salinities because continental waters seasonal changes (Stuardo and Martínez, 1975). The second one: Estero de Teacapan, where a mean salinity of 40 was present throughout: the year. This oyster population now acclimatized was originated as a consequence of an experimental oyster hatchery, whose seed were brought from Estero del Pozo, San Blas, Nayarit. (Fig. 1). The sample were frozen at -20 °C and transported to the genetics laboratory of the Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autonoma de Mexico, where theywere processed in the following way: ATTAINMENT OF HOMOGENATESThirty organisms from each population were handled as a representative sample. After defrost, the semitrasparent part of adductor muscle from each orgnism was removed; an homogeneus mixture was precticed in a buffer-Tris phosphate al 7.3 pH solution, during three minutes and centrifugated at 10,000 RPM fifteen minutes. ELECTROPHORESISElectrophoresis was worked in presence of Sodium dodecil-sulphate (SDS), following the Laemmli technique (Laemmli, 1970) on polyacrylamide 12.5% gel with SDS, into a discontinuos system based in both, a con centrator and another separator gel. The buffer solution within the electrophoretic chamber was prepared by a tris-mixture (1.52 gr), glicerine (7.21 gr) and SDS 10% (5 ml) disolved in 500 mi of water a 8.6 pH. Using a Hamilton syringe, 25 micorliters of sample per rail, which equivalence is 12.5 micrograms of protein were applied. Firstly switched at 50 volts, 11.3 m ampers until the sample went inside of the separator gel, secondly it was roce at 100 volts 13.8 m ampers. STAINING AND FIXINGThe gels were stained and fixed with a 25% isopropanol, 10% acetie acid and 0.02% blue Comassie solution durina two hours. DATA ANALYSISBy means of the computered statistical program of data analysis: BASIS (Burroughs Advanced Statistical Inquiry System), using the B7700 from Programa Universitario de Computo UNAM the dispersion and variability of each population was calculated. Histograms, the Student "t" test and the basic statistics mean, variance and standard desviation were also obtained. The different class intervals were analysed as the finest point of comparission to evaluate the variability of proteins with similar molecular weigth. Finally, assuming a variance homogeneity as a null hypothesis, the Student "t" test was made comparing the mean values between two samples for cach class interval. Data were arranged on tables and graphs looking for the difference between two populations and their correspondent places. (Tables 1, 2). RESULTSFor each population studied and their electrophoretic patterns it has been attained the following: The San Blas oysters population gas shown 17 bands, while the Teacapan is 14 (Figs. 2 and 3). 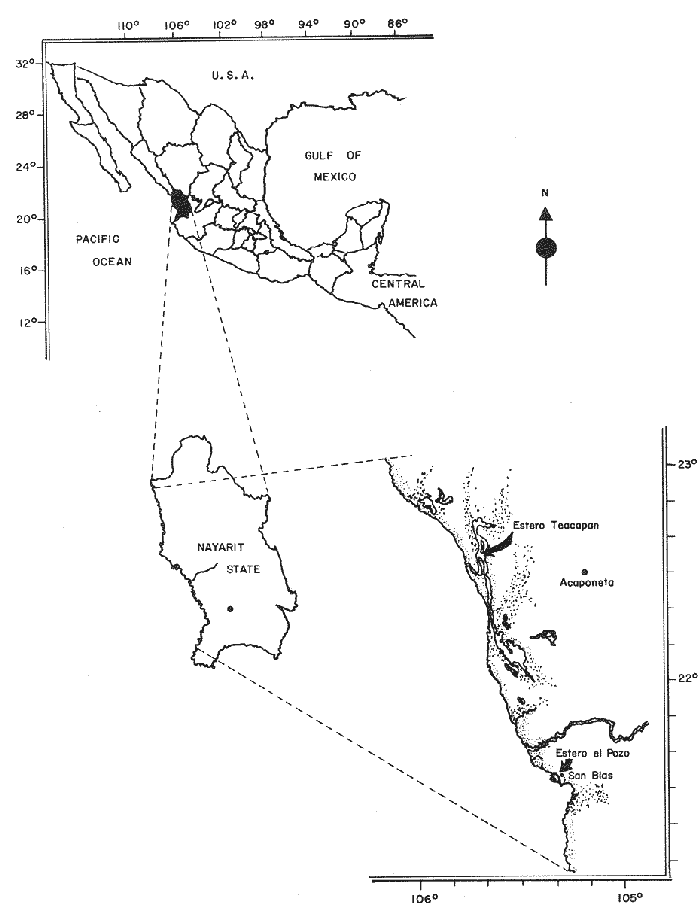 Figura 1. Map. of the locations where Crasstrea corteziensis was collected. 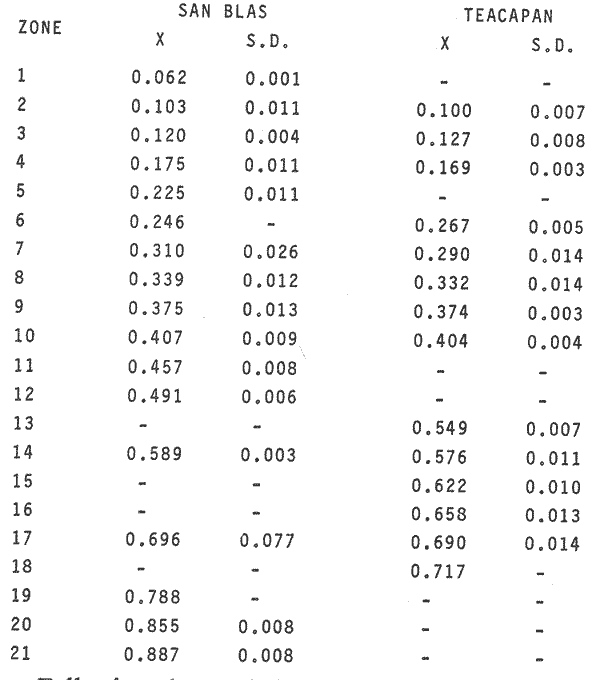 TABLE 1. MOLECULAR WEIGTH VARIABILITY PER ZONE IT IS DEMOSTRATED NO RELEVANT MOLECULAR VARIABILITY IN EACH ZONE OF THE TWO POPULATIONS HERE ANALYSED. 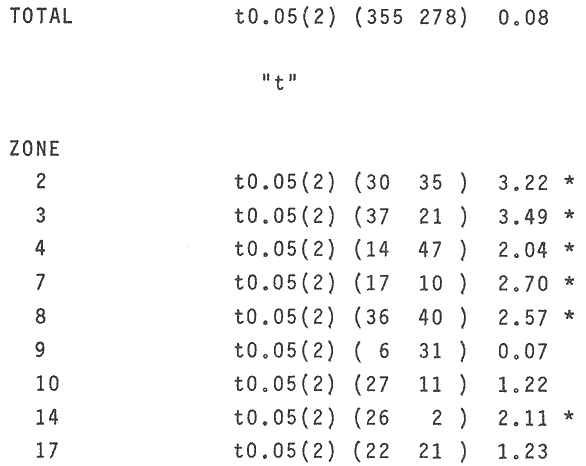 TABLE 2. RESULTS OF THE "t" TEST. COMPARISON OF MEAN VALUES PER ZONE AT 95 % CONFIDENCE LEVEL THE ASTERISKS INDICATIB REJEMON OF THE NULL HIPOTHESIS: * NO DIFERENCE EXISTS BETWEEN THE MEAN VALUES OF THE TWO POPULATIONS ASSUMING VARIANCE HOMOGENITY. Following thc statistical analysis, the San Blas population has shown a standard desviation and variation coefficient bigger than Teacapan ones. The Student "t" suggested that both populations had same origin. The zone by zone comparission indicated 3 strates, were the null hypothesis were not rejected, that is their mean values belonged to the same sample. Contrarily, six zones rejected the same hypotesis. DISCUSSIONOn the mexícan coasts, the family Osteidae is represented by the Ostrea, Crassostrea and Pygnodonta genera, not-withstanding the existence of the last one, is still hazardous. In agreement with the classic taxonomic criteria, 15 or 16 different oyster species appertaining to the Ostrea and Crassostrea genera have been recongnized in Mexico, nevertheless the plasticity of their conchs morphology is the main cause against this species description, which should take in account the existence or "ecological morphos", whose phenotypic expression works depending on the environmental qualities around them. In such manner, species have been confused and consequently their taxonomical and systematical position remains incertain (Mattox, 1949). The synonymous of Cassastrea coiteziensis may be an example of the formerly exposed. Synonymy: from Hertlein (Hertlein, 1951) 1. Ostrea chilensis; 2. Ostrea cibialis; 3. Ostrea longiscula; 4. Ostrea virginica; 5. Ostrea iridescens; 6. Ostrea coracoides; 7. Ostrea californica; 8. Ostrea englekyi; 9. Ostrea columbiensis As it can be seen there is confusion still at generic level. Due to the variability of both, the conch shape of adult oysters and the influence upon them of the environmental factors, researchers have been proposing other safety clues in oysters identification and classification like some larvarian conch features specially the umbo larvae (Forbes 1967). Other clues: the presence or ausence of the promial chamber, an anatomical characteristic trait of the Crassostrea genus (Nelson, 1938); the sexual habits, which in fact let us discriminate the no incubator species of Crassostrea from the larviparous Ostrea genus characteristically marine. 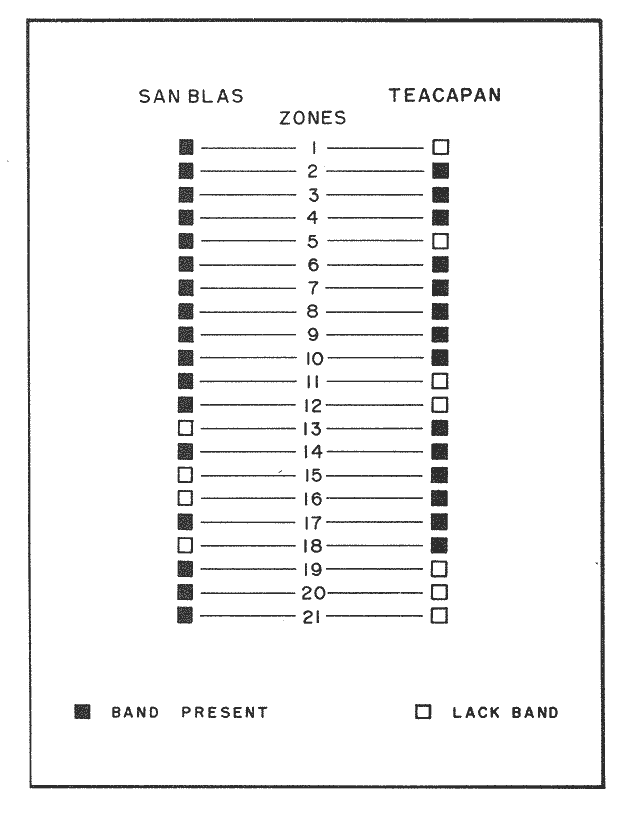 Figure 2. Protein band patterns in the two populations of Crassostrea corteziensis. However, the previously exposed characters have limited application and remain poorly considered by malacologists, specially those that are based on morphological conch features for their identification and classification. On the other hand, the number and the morphological choromosomic features have recently been used as a contribution to solve the taxonomical problem. In oysters like other organisms, the standard karyotypes represent the specific qualities on which the cytotaxonomy is supported (Ahmed and Sparks, 1967; Ahmed, 1973; Longwell et al., 1967; Menzel, 1968; Rodríguez Romero et al., 1979a, 1979b and 1981). 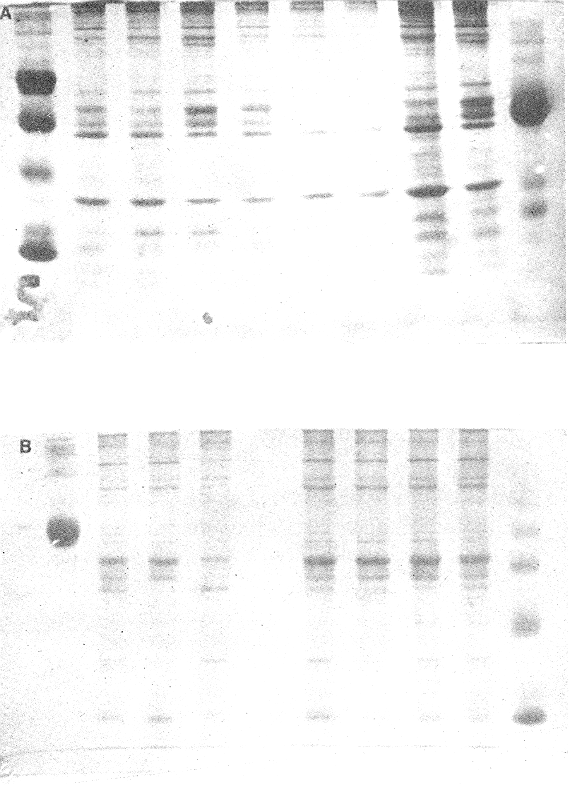 Figure 3. Electrograms of the two populations of Crasowea corte zimus; A. Estero de Teacapan. B. Estereo del Pozo The cytogenetic contributions about the most common oyster species in the mexican coast may be summarized as follows: 1. The Ostreidae family and its three genera have shown through their studied species, a 20 (2n) invariable chromosomic numbcr. There is a wide karyotypic stability without sexual chromosomes. 2. Phylogenetically, the species are closely related in such a manner that from the cytogenetical and cytotaxonomical point of view, they cannot be recognized a different species. Any way karyotypes of each species presented their characteristical traits well defined. Otherwise, the free aminoacids chromatographic profiles from different species of the Ostrea and Cassostrea genera have been studied and have reflected parallel similitudes and differences that lay in concordance with the results of studies of external morphology, anatomy and cytogenetical analysis. Therefore, it can be drawn clearly each species features (Hillman, 1964; Rodríguez-Romero, 1981). The electrophoretic results here reported are an attempt in the initial stage of the genetical expression at protein level research, towards the discernment of the same species but with different population peculiarities. These subjects must be focused in the understanding of Crassostrea corteziensis adaptive behaviour, in order to sketch its evolutionary and phylogenetical traits within the Ostreidae family. By comparing statistically the protein mobility (PM) of the electrograms sampled, it was not found significative difference. Not-withstanding, the findings per zone have shown in San Blas oyster population a greater protein diversity as a net difference between thege studied populations. This may be explained by the adverse environmental conditions as hipersalinity and the shallow water antiestuarine stratification beings in Teacapan. So, this adaptation of oysters population, can be expressed at electrophoretic patterns, level, reflected by the lack of three protein types in the acclimatized oysters first generation. However, these conclussions must be revised and supported by means of procedures to measure the variability, like the allelic numbers for different enzymes, at populations and interspecific level. On this way, it could get an incipient evidence of a probable cladogenetic process. It is recomended fecundation and interbreeding experiments with distinct progenitor populations and with other oyster species and their analysis by cytogenetic and biochemistry-genetical procedures. The whole study bring us to a clearest understanding of the adaptive process and of the ecological situation of these populations which must be considered in some stage of their evolutive process. LITERATURAAHMED, M. and A. Y, SPARKS, J. Fish Res. Bd. Cam, A preliminary study of chromosomes of two species of oysters (Ostrea lurida and Crassostreas). 1967. 2155-2159. 24: AHMED, M., Cytologia, Cytogenetic of Oysters. 1973, 337-346. 38 (2): BUROKEI; N. E.; W. Y, HERSHBERGER and K.K, CHEW, Mar. Biol., Population geneties of the family Ostreidae. 2. Interespecific studies of the genera Crassosirea and Saccostrea. 1979. 171-184. 54 (2): FORBES, M. L, Bull. Mar. Sci, Genetic differences in prodissoconchs of Gulf of Mexico Oysters. 1967. 338-347. 17 (2): FUJINO, K. and N. NAGAYA, Bull Jap. Soc. Sci. Fish., Biochemical polymorphism in the Pacific Oyster. I. Variants in Myogin and esterases. 1977. 938-988. 43 (8): HERTLEIN, L. G., Bull Cal. Acad. Sci. Description of two new species of marine pelecypods from west Mexico. 1951. 68-75. 50 (2): HILLMAN, R. E., Syst. Zool., Chromatographic evidence of intraspecific genetic differences in the castem oyster Crassostrea virginica. 1964. 12-18. 13 (1): JOHNSON, A. G., F. M. UTTER and Y, NIGGOL, Anim. Blood Groups. Biochent. Genet., Electrophoretic variants of aspartate aminotransferase and adductor muscle proteins in the native oyster (Ostrea lurida). 1972. 109-113. 3 (2): LAEMMLI, U. K. Nature, Cleavage of Structural proteins during the assembly of the head of bacteriophage T4. 1970. 680-685. 227: LONGWELL, A. C., S. S. STYLES and D. G. SMITH, Can. J. Genet. Cytol, Chromosome complement of the american oyster Crassostrea virginica as seen in meiotic and eleaving eggs. 1967. 845-856. 9 (4): MATTOX, N. T., Ecol. Monogr., Studies on the biology of the edible oyster, Ostrea rhizophorae Guilding, in Puerto Rico. 1949. 339-356. 19: MORE, P., M. T. MORE and J. POISBEAU, Bull. Soc. Pharm. Quest., Aplication de la methoda de disc - electrophoresis a l'etude de proteins solubles des muscle adducteurs de Ostrea edulis L, Crassostrea angulata Link, tapes de cussatus Mytilus edulis et Mya arenaria L. 1966. 135-142. 8: MORE, P., M. T. MORE, M. R. MONNET and J. POISBEAU, Interet taxonomique. C. R. Acad. Sci. Paris, Ser. D, Electrophorese en gel de polyacrylamide des proteines solubles de la partie transparente du muscle adducteur de cinq especes d'ostreidae. 1971a. 222-225. 273 (2): MORE, P., M. T. MORE, M. R. MONNET and J. POISBEAU, C. R. Herb. Seances Acad. Sci. Ser. D. Sics. Nat., Concerning the adenylate kinase of the soluble proteins of the adductor muscle of the oyster. 1971b. 1331-1334. 273 (15): NELSON, T. C., J. Morph, Tle feeding mechanism of the oyster. I. On the pallium and branchial Chambers of Ostrea iliginica, O. edulis, and O. angulata, with comparisons with othcrspecies of the genus. 1938. 1-61 63: RODRÍGUEZ-ROMERO, R., M. URIBE and A. LAGUARDA, Venus, Cyto-genetic study of an oyster population of the species Crassostrea virginica from the coast of Tabasco, Mexico. 1978. 83-86. 37 (2): RODRÍGUEZ-ROMERO, F., A. LAGUARDA and M. URIBE, An. Centro Cienc. del Mar y Limnol. Comparative analysis of the karyotypes of two oyster species of the genus Crassostrea from Mexico: C. virginica and C. corteziensis. Univ. Nal. Autón. México, 1979. 19-24. 6 (1): RODRÍGUEZ-ROMERO, F., M. URIBE and A. LAGUARDA, An. Centro. Cienc. del Mar y Linmol. The karyotype of Crassostrea corteziensis Hertlein (Mollusca-Ostreidae). Univ. Nal. Autón. México, 1979b. 15-18. 6 (1): RODRÍGUEZ-ROMERO, F., Tesis Doctoral Facultad de Ciencias, Estudios cromosómicos y de expresión génica en moluscos bivalvos de importancia económica de las costas mexicanas. Univ. Nal. Autón. México. 1981. 122 p. STENZEI, H. B., Oysters. In: Treatise on Invertebrate Paleontology. Part N. Bivalvia. V.3. Mollusca G. Univ. Kansas/Geol. Soc. Amer., Inc., Boulder, Co (USA). 1971. 272 p. STUARDO, J. and A. MARTÍNEZ., An. Centro Cienc. del Mar y Limnol. Relación entre algunos factores ecológicos y la biología de poblaciones de Crassostrea corteziensis H. 1951 de San Blas Nayarit, México. Univ. Nal. Autón. México, 1975. 89-129. 2 (1): THOMPSON, J. M., Austr. J. Mar. Fresh wtr. Res., The genera of oysters and the Australian species. 1954. 132-168. 5 (1): TORIGOE, K. and A. INABA, Venus, Electrophoretic studies on some oysters. 1975. 177-183. 33 (4): TORIGOE, K., Venus, Electrophoretic variants of adductor muscle proteins in Crassostrea gigas. 1978. 241-244. 37 (4): WILKINS, N. P. and N. F. MATHERS, Anim. Blood Groups Biochem. Genet., Enzyme polymorphisms in the european oysters, Ostrea edulis L. 1973. 41-47. 4 (1):
|

