|
DIET, FOOD CONSUMPTION AND GROWTH OF THE DAB Limanda
limanda (LINNAEUS) IN ISLE OF MAN WATERS, UK
Trabajo recibido el 4 de
septiembre de 1985 y aceptado para su publicación el 15 de febrero de
1988.
A.A. Ortega-Salas
Universidad Nacional Autónoma de
México. Instituto de Ciencias del Mar y Limnología, Ciudad
Universitaria, 04510 México D.F. Contribución 608 del Instituto
de Ciencias del Mar y Limnología, UNAM.
En el contenido estomacal de "dabs" se encontró
una gran variedad de comida. Los especímenes encontrados con mayor
frecuencia se atribuyen a que son más abundantes en el área, en
ese momento, en lugar de ser un tipo de comida preferencial. La clase de comida
ingerida sugiere que los "dabs" comen durante las horas del día. El peso
promedio del tracto digestivo mostró que los "dabs" se alimentan
más en verano que en invierno, lo que se refleja en el crecimiento del
pez. Se analiza un método para calcular el consumo de comida diario.
Esto dio una estimación del consumo de comida de las existencias de dabs
en el mar de Irlanda en 1977 de 66,000 toneladas, y la eficiencia de
conversión fue de 5% hasta peces que pesaron 200 g. La relación
de comida ingerida respecto al peso del cuerpo fue de 4% en todas las
edades.
A wide variety of food type was found in the stomach
content of dabs. Food items more frequently found was atribbuted to be more
abundant in the area rather of being of food preference. The kind of food
intake suggested dab eats during day light. The average weight of the digestive
tract showed that dab feeds more in summer than in winter. This is reflected in
fish growth. A method to calculate daily food consumption is analyzed. This
gave an estimate of food consumption of the Irish Sea dab stock in 1977 of
66,000 tonnes and the efficiency of conversion was about 5% up to 200 g fish
weight. The relation of food intake to gutted body weight was around 4% at all
ages.
An important aspect of the ecology of fish population is the study of diet, food consumption and growth. The volume of food available and/or the metabolism of the fish may vary seasonally such that behaviour and feeding habits follow a specific pattern. Diet and feeding habits of fish have been described by Jones (1952; Edwards and Steele, 1968 and de Groot, 1971). Hynes (1950) has reviewed commonly used to analyze stomach contents. The aims of this study are: 1. Identify food type. 2. Examine feedings, behaviour. 3. Describe seasonal variation. 4. Measure food intake and total food consumption in the Irish Sea during 1977.5. Relate food intake to body weight. MATERIAL AND METHODSDabs were caught around the coastal of the Isle of Man between 54° 00' and 54° 25'N, and 4° 55' and 4° 10'W in depths from 4 m - 40 m. A few additional samples were taken 20 miles E of the Island at a depth of 20 m - 40 m, and 3 miles SE of the Island between 30 m - 50 m. Monthly samples were taken, weather permitting, from November 1976 to March 1979 by means of an otter trawl with wings of 70 mm mesh size and cod end of 45 mm. Weekly samples of young fish were taken in Port Erin Bay at depths of 4 - 8 m whenever possible from April 1977 to May 1979, using a beam trawl with mesh size of 60 mm exterior net, and 15 mm interior lining net. A total of 4,329 dabs from all sources were examined. Dabs which were not processed within 24 hs of capture were frozen for no more than three to four weeks. Trawled fish were measured to the nearest mm and weight to the nearest g. Guts and gonads were dissected out until further analysis. Throughout a year, food items were identified and counted from 220 digestive tracts (Tables 1 and 2). Age at capture was reduced from the number of opaque (summer) bands and hyaline (winter) rings on the otolith. A hatched in May was assumed for all dabs and the age was recorded in months. 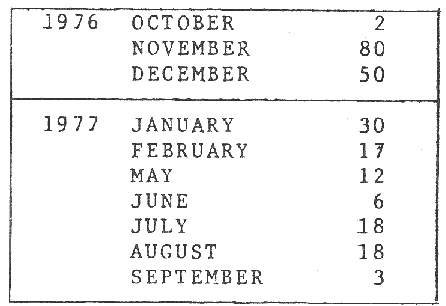 TABLE 1 SAMPLES ANALYSED Records of each fish were maintained as a computer file. The results were processed in the ICL 1906A computer from the University of Liverpool and the CD 7600 from the University of Manchester the Statistical Pack age for the Social Sciences version 5 and 6, respectively. Scattergrams represented individual data points by mean of an asterisk (*); more than one, in the same position, were marked by the number of fish to a maximum of 9 but all the data were used for calculations. Scales differed on a given pairs of scattergram for males and females, as the feature of the computer program employed an automatic scale for the data provided. Lines showing maximum values were fitted by eye. Other graphs than scattergrams the symbols (X) represented one specimen and (0) mean that 0.95 CI.I. were too big to plot. 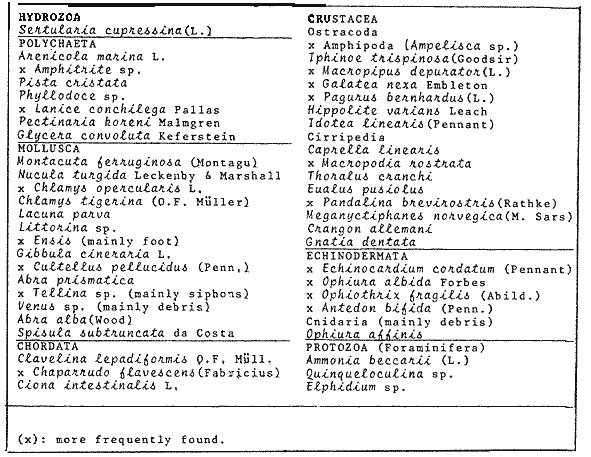 TABLE 2 FOOD ITEMS IDENTIFIED RESULTS
FOOD TYPESampling was usually carried out at midday. A wide variety of food items was found listed in Table 2. Identification was made by means of recognizable body parts e.g. rostrum of decapods, thorax of crabs, siphons and foot of mollusca, legs of ophiurida, debris of shall, etc. Although dab may have some preference for food in both summer and winter, it is likely that feeding activity is partly related to day length and the time taken for digestion of the food. Digestion takes longer in winter than in summer, probably because of the effect of differences in sea temperature on the fish. Around the Isle of Man sea temperature varies from about 7°C in winter to 14°C in summer (Ortega-Salas, 1980). In dab from the Irish Sea, Jobling et al. (1977) found that lowering the experimental temperature from 16.4°C to 8.5°C markedly increased gastric evacuation time, which varied from about one to two days depending on the size of the fish and size of the food. FEEDING BEHAVIOURPersonal experience in the laboratory showed that when a group of young fish within an aquarium was well fed in the morning, in the afternoon they required more food and in optimal conditions they hardly stopped feeding. However, in natural conditions fish arc adapted to live where food is available. It is assumed that in the wild each special of fish lives where the substrata and/or water temperature and/or salinity are within the limits of the species tolerance. Brander (1980) found in the Irish Sea that dab were more abundant in muddy sand and Jones (1952) showed that in the Cumberland Coast dab were more abundant in muddy and fine sand (where most of their food items live). There are many factors which act on the growth of individual fish which do not permit one to generalize about any population sampled. However it was assumed that taking the average amount of food in the stomach of sampled fish gave an indication of food intake by the population at each age. Full stomachs indicated that food was readily available and conditions suitable for feeding. While empty stomachs possibly indicated that there was not enough food or the temperature was too low. Steven (1983) suggested that comparisons of the food intake reflect feeding habits of species. In Isle of Man waters, the stomachs of dab were frequently found to contain parts of animals including chelas, ophiurida legs, feet of mollusca, parts of annelida, siphons of bivalve, thorax with legs of Crangon, small crustacea together with parts of seaweeds and several times, pebbles cand sand (containing foraminifera). These findings indicate that dab are visual feeders and bite parts of big prey (although small prey are usually easier to hunt). Observations of two dab from age-gp 1 (125 mm) within an aquarium showed that they actively bite and suck at the food provided. They used their body power to pull pieces off from the food while swimming. East of the Isle of Man on the Cumberland Coast, Jones (1952) found that plaice and dab were both visual feeders. The dab were more active than plaice and took a wider variety of organisms. Sole on the other hand were tactile feeders and were almost independent of vision for finding and recognising thier food. Burroweing polychaetas were taken by plaice to a greater extent than by dab: while dab ate more of the epifauna, such as Aphrodite, Eupagurus and Philine. Jones also showed that the number of fish feeding in winter was much lower than in summer. He found that dab sampled in muddy sand, had eaten in order of importance: Amphiura filiformis, Cultellus pellucidus, Philine aperta, Aphrodite aculeata, Eupagurus bernhardus, Ampelisca spp and Pectinaria spp, and in fine sand they had eaten: Abra alba and Ensis ensis. In the same kind of substrata around the Isle of Man more Cultellus pellucidus were found in the stomachs but no Amphiura filiformis. More Ophiura albida and Ophiotrix fragilis were found which could be related to the different grounds from which the samples were taken. It was felt that observations of dab within an aquarium would be useful in order to understand their natural behaviour. An aquarium was set-up with running water from the sea and a sand bed (collected from the beach). It also contained a sponge, a Coelenterata (Plummularia) a Chordata (Tunicate) (Clavelina lepadiformis), three specimens of Ophiurida, tow mollusca (Pulmonata), four small crabs (Portunus), two terebelidae (Polychaeta), one Chlamys and one Pecten (all live). The two dabs (125 mm) fed very well on small crustacea, which came from the inflow water, but they mainly fed on pieces of chopped queenies given as food. The dab did not feed on any of the surrounding animals, although at time, starved for over 2 days. When the crabs grew larger (from 1 to 4 cm) they were seen chase the dab. Usually the dab were active at night and during the day buried themselves for long periods under the sand to a depth of 1 to 2 mm. The female dab grew an average 9 mm per month in length and 5.6 g per month in weight. The male grew 8.6 mm per month in length and 5.8 per month in weight. The fish were kept from October to January at 14 - 16°C (although at weekends temperature dropped by up to 5°C). The two dab lived for five months in the aquarium, but both had physical injuries to the mouth caused by struggling to escape. From these observations it is difficult to understand why the dab did not feed on the surrounding animals (which are their natural food items). It is likely that the stress of being enclosed changed the natural behaviour and that the food given was more accesible. The wide range of food items in the stomach together with observations in the aquarium, show that dab are visual feeders. They appear to feed more on readily available food items than specific ones. Although, in the wild, the temperature decreases from October to January and fish growth diminishes considerably, the dab in the aquarium continued growing almost at the same rate as in summer. They were well fed and temperature was similar to the summer. However one must be cautious in generalising from behaviour observed in the aquarium to behaviour in the wild, as the fish did not appear to adapt to the control conditions of the experiment. SEASONAL VARIATION ON FEEDINGThe aim of this section is to find any seasonal pattern of variation in feeding. So the wet weight of the stomach and intestine with contents was taken as an index of food intake (which for brevity was "stomach weight"). It has been stated through the fisheries literature that in temperate waters fish usually feed vigorously in summer and more slowlly in winter. This could be a reach to believe that food preferences of the dab differ between summer and winter. Lands (1976) recorded that polychaetes were dominant in the digestive tract of dab in late autumn and in winter in North Trondelag. Norway, Mollusca attained their peak in March and ascidians were more frequent in autumn. Lee (1972) in the North Sea and Jones (1952) on the Cumberland Coast (eastern Irish Sea) found that the dab did not show any seasonal variation of diet. However all these workers found the dab eat a great variety of food items. Todd (1907) suggested that there was evidence to show dab in the North Sea did not feed as much during winter (Nov-Mar) as during the remaining months of the year. Analysis of gut contents showed that in winter many stomachs were empty and those of feeding fish were about half full. In summer most of the fish were feeding, so the guts were about half full to full. During November in Port Erin Bay some fish had eaten a lot of Iphinos trispinosa (Goodsir) (Cumacea) and the same month in Laxey Bay other contained many Idothea linearis (Pennant) (Isopoda) holding eggs. In another sample a female dab (308 g in weight) contained 44 Ophiura albida Forbes (Echinodermata) whose discs were about 8 mm in diameter and some pebbles of about 15 mm in diameter. This may indicate that dab eat the food items which are readily available in the area rather than maintained a specific diet. Figure 1 shows mean stomach weight plotted against age of fish at two monthly intervals throughout the year. Figure 2 shows the actual distribution of these data in two dimensions. It is clear from figure 1 that there is an annual cyclic variation with an average increase in weight as fish grow older. These annual cyclic variations show that empty or partly filled stomachs were commonly observed in winter, but in summer there were fewer empty stomachs and most of the stomachs were full. In figure 1 the curve shown is the average of the overall stomach and intestine weight with contents. This curve was calculated from predicted lengths of the fish at time t (Lt) together with the coefficient of the ln stomach weight - LN length relationship (Fig. 3) in order to calculate the average stomach weight at time t (Ortega-Salas, 1981a) as follows: 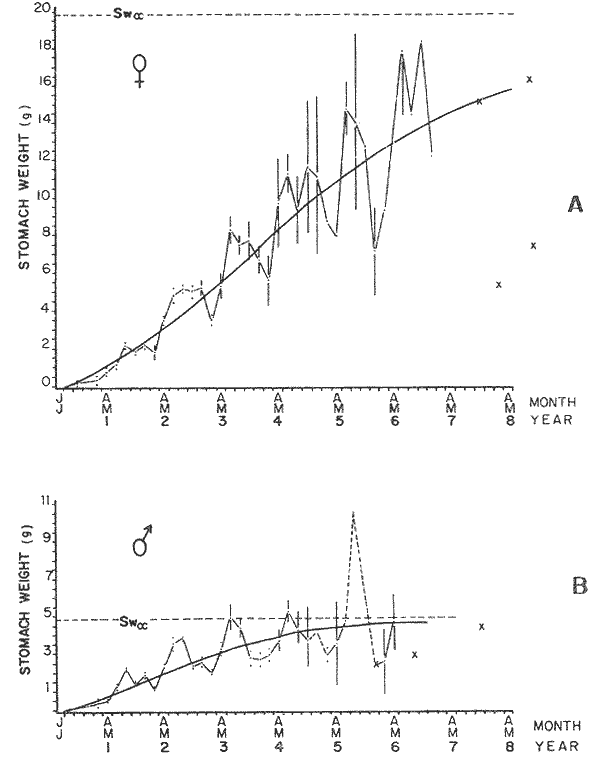 Figure 1. Mean stomach weight for age at two monthly intervals with 0.95 C.I. Theoretical curve: A= females: stomach Wtt= 0.00000108 Length mmt 2.883; B= males: stomach Wtt= 0.00000238 Length mmt 2.691 If Lt = Lα (1-e-k(t-to)) (von Bertalanffy's) and Sw = a Lb; Swt = a Ltb where Lt = total length at time t; Swt = "stomach weight" at time t. The theoretical equation which describe the curves are as follows: FEMALES: In "stomach weight"gt = 2.883 (ln length mmt)-13.7307 MALES. In "stomach weight"gt = 2.691 (ln length mmt)12.9476 FEMALES: R = 0.89, n = 2623; MALES: R = 0.79, n = 1705 Which could be described as follows: FEMALES: "Stomach weight" gt = 0.00000108 length mmt2.883 MALES: "Stomach weight" gt = 0.00000238 length mmt2.691 The pattern of seasonal variation of feeding (using the "stomach weight" and observations during the present study) shows that dab feed more in summer than in winter. The calculated curve (von Bertalanffy's) gives an estimate fo the average "stomach weight" (stomach and intestine weight with food intake) at each age within the oscillation. 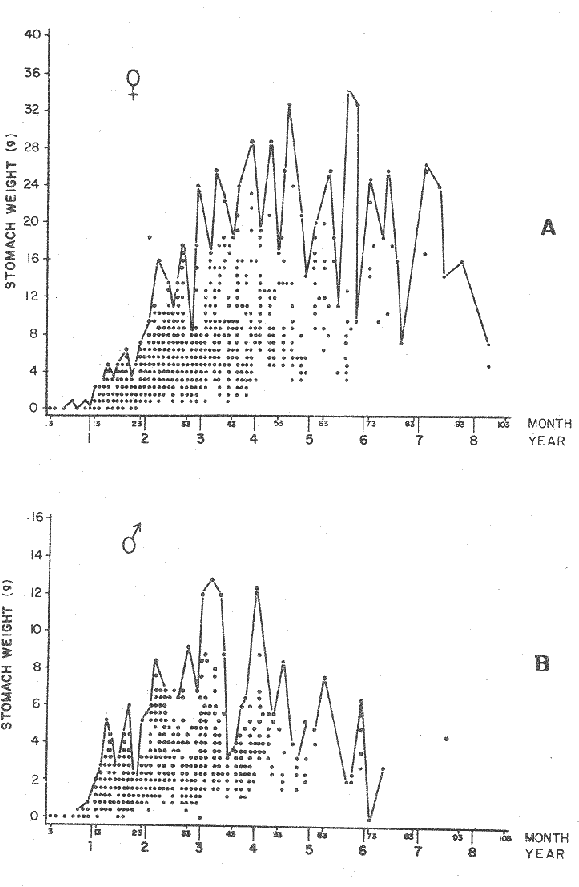 Figure 2. Stomach weight for age at monthly intervals. A = females; B = mates. ESTIMATE OF FOOD CONSUMPTIONThe oscillation of the mean weight of the stomach and contents (Fig. 1) shows the absolute food intake at each age. The lower points of the oscillation are considered to represent the weight of the stomach and intestine itself while the higher points show the increment with food. In order to estimate the dialy food intake of dab at age, an assumed growth curve for stomach and intestine weight only was first plotted to the lower values of the oscillations (Fig. 4). The growth curve for stomach and intestine itself was calculated from the In "stomach weight" - In Fish length relationship using data from February and March, when the fish stomachs were relatively empty (winter feeding level). Using the calculated fish length at the end of the age-gp from the von Bertalanffy model, stomach weight was calculated for each age (St = a Ltb). The predicted curves for stomach and intestine are given by the following equations: FEMALES: St = 0.0000018 Lt2.71724 MALES: St = 0.000003746 Lt3.53341 where: St = Stomach and intestine weight (g) at time t; Lt = Fish length(mm) at time t. The difference between this line and the oscillatory curve indicates the average daily food intake. This average daily food intake for each two months period was then multiplied by 61 days to estimate the average amount of food eaten in two months. The addition at six consecutive two months period indicates the amount of food a dab eats yearly (Table 3). Using Table 3 the quantity of food the fish eats at each age may be calculated an related to the corresponding length and weight estimate. The weight (W) and the gutted weight (GW) were estimated from the following equations (Ortega-Salas, 1981a, 1981c): GUTTED: Females: GWt= 0.00000545Lt3.1001. Males: GWt= 0.00000526Lt3.1076 WHOLE Females: Wt = 0.00000495 Lt3.1396 Males: Wt = 0.00000566 Lt3.1091 Table 4 and figures 5, 6, 7 and 8 show that (Fig. 5) female dab from age-gp I and age-gp VI eat in a year approximatelly 250 g and 2000 g of food respectively. A male dab at the same ages eats about 238 g and over 90 g of food. Figure 8 female dab require from 500 g to 2000 g of food yearly from age I to VI. Males from I to III require from 400 g to over 900 g of food. Figure 7, the mean efficiency of the fish to convert food to growth (weight) is 5% up to age IV and V (200 g fish weight). Figure 8 the efficiency fo conversion (AW/F or AGWT) decreases with age from 11% (male) and 1% (female) at age-gp I to 1% at age-gp VI (both sexes). Jones and Hislop (1972) showed that haddock and whiting (demersal fish) captured in the North Sea maintained in aquaria for about 35 days at 13°C to 14°C gave an efficiency of conversion of squid to growth (weight) 20 - 30% for both species. Hence we may suggest that fish in natural conditions have to eat 4 to 7 times more in order to gain the same unit of weight. This is not surprising as the wild fish has to use energy to find food, escape from predators and migrate, either to better conditions or for reproduction. 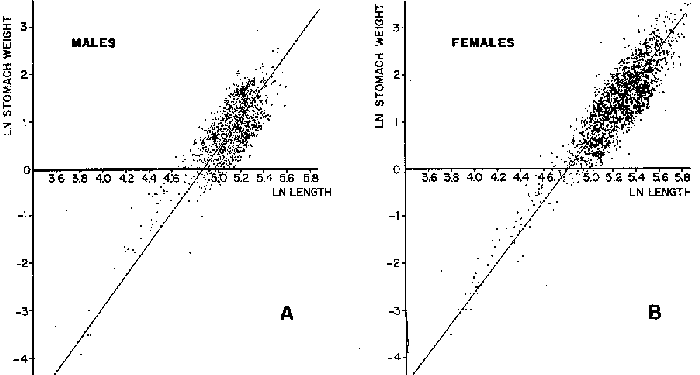 Figure 3. Individual In stomach weight plotted against In fish length. A = Males: Sw(g) = 2.691 (In L(mm)-12. 9476); b = Females: SW(g) = 2.883 (in L(mm) 13.7307) 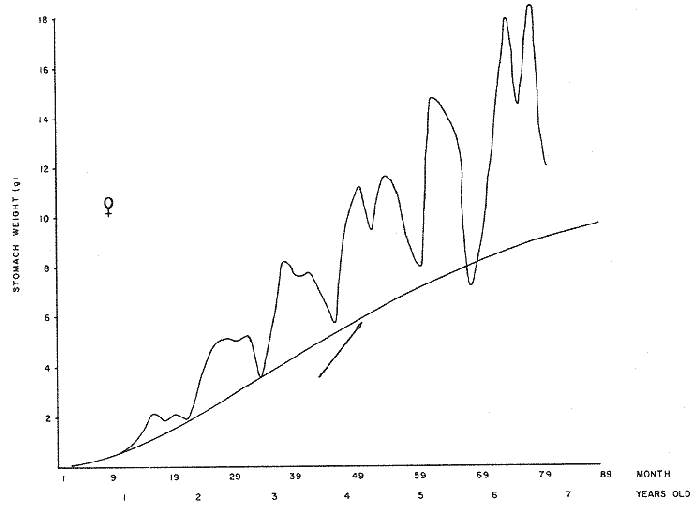 Figure 4A. Mean stomach weight for age. The arrow shows the theoretical curve of stomach and intestine (assumed empty of food). Females: St = 0.0000018Lt 171754. 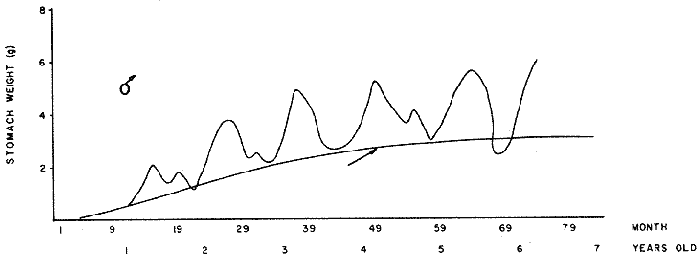 Figure 4B. Idem 4A. Males: St = 0.000003746Lt2.53341. Using the results Table 4 together with the estimate catch and stock number for the Irish Sea in 1977 (Ortega-Salas, 1981a, 1981d) it is possible to calculate total yearly food consumption by the dab population in the area (Table 5). Approximately 66,000 tones of food (21,954 t for males and 43,877 t for females) would be required yearly to maintain the stock with the present population structure. In order to determine the % of "stomach weight" (as index of food intake) with respect to the gutted fish at each age, a plot was made showing individual % stomach weight to the gutted weight at each age (Fig. 9) and also the average respective for the age (Fig. 10). The ratio of "stomach weight" to the fish weight was found to be constant throughout the age-gps. This follows the allometric relationship of an any organism, although there is a negative slope tendency as fish get older; which could be because the bigger fish (at older ages) are missed in the samples (Fig. 2). Scattergrams (Fig. 9) show this negative tendency, which is stronger in males, although may be considered to be proportional at all ages. It is clear from figure 10 that there is an average of 4.7% of stomach and intestine (itself) relative to the gutted weight for females and about 4% for males. Above this, there is an oscillation of food intake, relative to the gutted weight at each age of about 4% which shows clearly there is greater food intake in summer than in winter. Using the whole weight those percentage calculated from gutted weight are not very different. 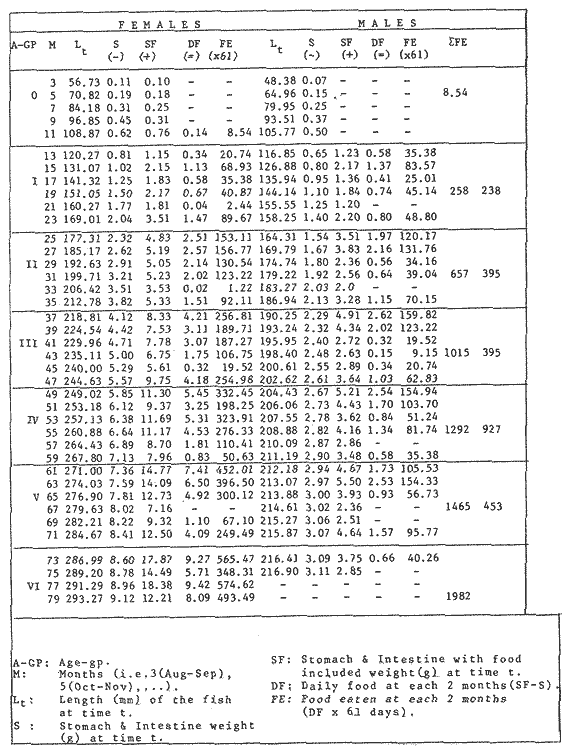 TABLE 3 ESTIMATE OF DAILY FOOD INTAKE FOR DAB AT EACH AGE. A-GP= age-gp; M= months (i.e. 3(Aug-Sep) 5(Oct-Nov); Lt= length (mm) of the fish at time t; S = stomach and intestine weight (g) at time t; SF = stomach and intestine with food included weight (g) at time t; df = daily foodat each 2 months (SF-S); FE= food eaten at each 2 months (DF X 61) Thus, the results may be summarized as follows: 1. The variety of food type found in the stomach content was wide, it is suggested that the dab did not have any particular preference for food and that the food items more frequently found were probably more abundant in the area. 2. The kind of food intake gave an index of the feeding behaviour, which suggests that dab eat during day light (which also could be another reason of why they actively eat in summer). The behaviour observations in the aquarium gave some support to these conclusions. 3. The average weight of the digestive tract (including food) gave a good index of seasonal variation on feeding showing that dab feed more in summer than in winter. This variation gave a clue to estimate food eaten at age. 4. An estimate of food consumption for the Irish Sea dab stock, in 1977, was 66,000 tonnes and the efficiency of conversion was about 5% up to 200 g weight of fish. 5. The relation of food intake to gutted body weight was around 4% at all ages, this clearly shows, that fish eat more in summer than in winter. 6. The estimate of average food intake gave (as a result) the average rate of growth estimated for the population of dab sampled. 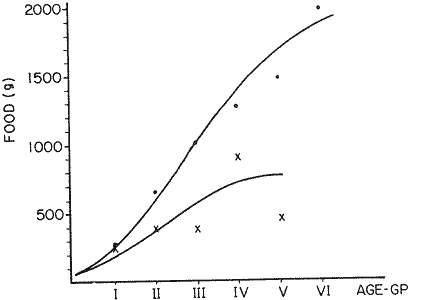 Figure 5. Estimate of quantity of food (g) eaten by a dab at each agegp. Females (.), Males (x). Lines were fitted by eye. 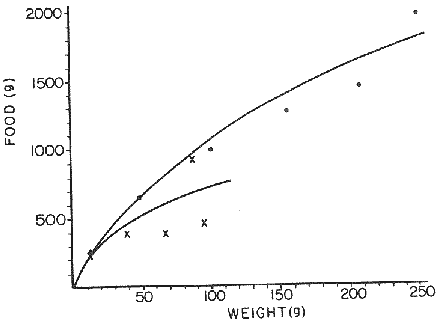 Figure 6. Estimate of quality of food (g) required by a dab in relation to its weight (g). Females (.), Males (x). Lines were fitted by eye 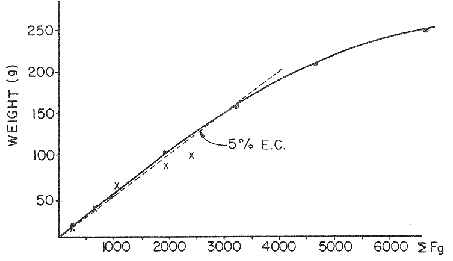 Figure 7. Fish efficiency to convert food in growth (weight). Females (.), Males (x). Lines were fitted by eye. 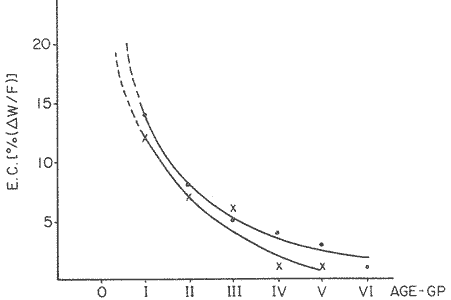 Figure 8. Efficiency of Conversion (E.C.) expressed as % of the relation increment of weight (Delta W)/food eaten (F) at each age-gp. Females (.). Males (x). lines were fitted by eye. 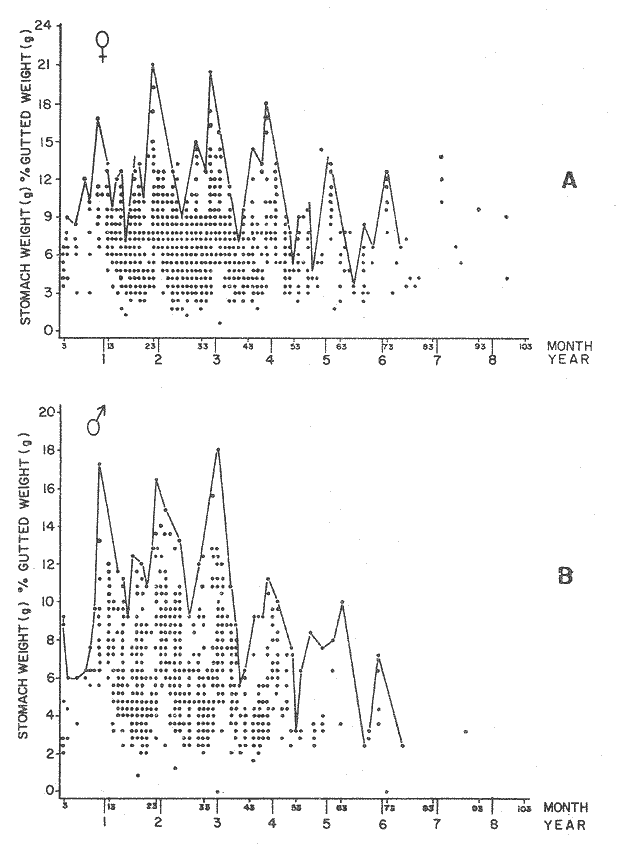 Figure 9. Stomach weight as % of the gutted weight forage at monthly intervals. A = Females; B = Males. 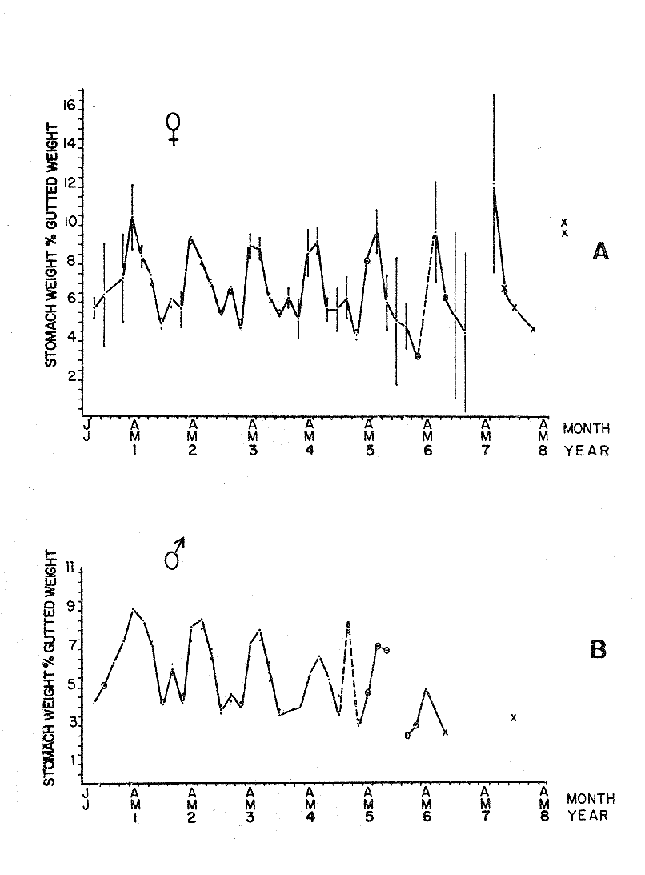 Figure 10. Mean stomach weight as % of the gutted weight for age at monthly intervals with 0.95 C.I. A = Females; B = Males. 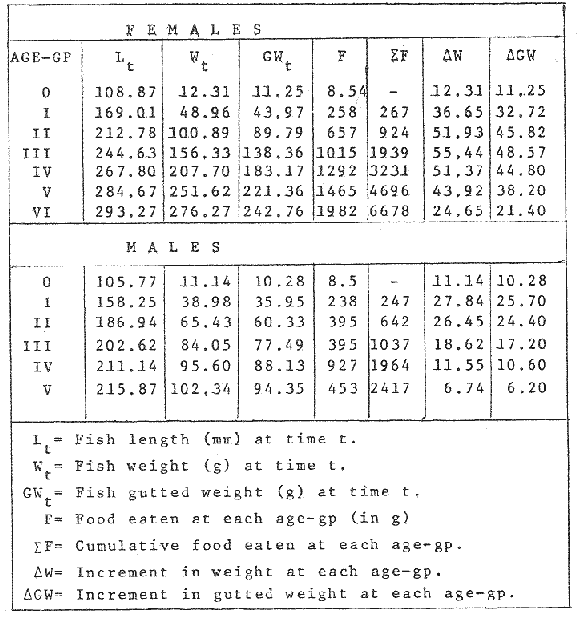 TABLE 4 FOOD EASTEN ESTIMATES WITH LENGTH AND WEIGHT AT AGE 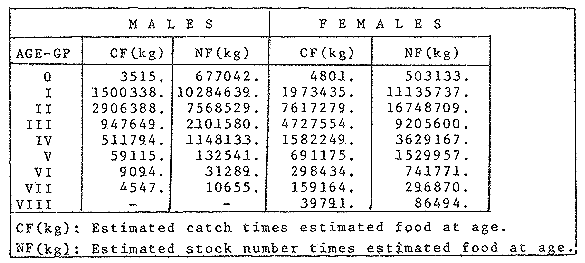 TABLE 5 ESTIMATES OF FOOD CONSUMPTION BY DAB POPULATION IN THE IRISH SEA IN 1977 AgradecimientosAcknowledgements are due to L. Birkett, P Bromley, and K. Sullivan for their advise. For laboratory facilities, to Department of Marine Biology of the University of Liverpool, and MAFF. Fisheries Laboratory, Lowestoft. And for the finantial support, Universidad Nacional Autónoma de México: Consejo Nacional de Ciencia y Tecnología; and the British Council. LITERATURABRANDER, K. Protection of Life in Sea, HeIg. Meer., Fisheries management and conservation in the Irish Sea. In: Kinne, O. and H.P. Bulnheim (Ed.). 1980. 657-599. 33 (1-4): EDWARDS, R. R. and J. H. STELL, J. Exp. Mar Biol. Ecol, The ecology of 0-group plaice and common dabs at Loch Ewe. 1. Population and Food. 1968. 215-238. 2: GROOT, S. J. de, Neth. J. Sea Res., On the interrelationship between morphology of the alimentary tract food and feeding behaviour in flatfishes (Pisces: Pleuronectiformes). 1971. 121-196. 5 (2): HYNES, H. B. N., J. Anim. Ecol. The food of freshwater sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in the studies of the food of fishes. 1950. 36-58. 19: JOBLING, M., D. GWYTHER and D. J. GROVE, J. Fish. Biol., Some effects of temperature, meal size and body weight on gastric evacuation time in the dab, Limanda limanda (L.). 1977. 291-298. 10: JONES, N. S., J. Anim Ecol., The bottom fauna and the food of flatfish off the Cumberland Coast. 1952. 182-205. 21: JONES, R. and J.R. HISLOP, J Con& Int. Expl. Mer., Investigations into the growth of Haddock, Melanogrammus aeglifinus (L.) and Whiting, Merlangilus merlangus (I.) in aquaria, 1972. 174-189. 34 (2): LANDE, R., Norway J. Zool., Food and feeding habits of the dab (Limanda limanda (L.)) in Borgenfjorden. North Trondelag, 1976. 225-230. 24: LEE, C. K. C., Ph. D. Thesis The biology and population dynamics of the common dab, Limanda Iimanda (L.) in the North Sea. Univ. East Anglia. UK. 1972. 105 p. ORTEGA SALAS, A. A., J. Fish. Biol., Seasonal changes in the common dab, Limanda limanda (I.) in Isle of Man waters. 1980. 75-82. 16: ORTEGA-SALAS, A. A., Ph. D. Biology of the dab, Limanda limanda (L.) in Isle of Man waters. Univ. Liverpool. UK. 1981a. 98 p. ORTEGA-SALAS, A. A., An. Inst. Cienc. del Mar y Lintnol. Age and growth of the Dab Limanda limanda (L.) otoliths in Isle of Man waters. Univ. Nal. Autón. México, 1981b. 69-78. 14 (1): ORTEGA-SALAS, A. A., An. Inst. Cienc. del Mar y Limnol. Age and growth to the Dab, Limanda limanda (L.) in Isle of Man waters. Univ. Nal. Autón. México, 1981c. 15 (1) 1988. ORTEGA-SALAS, A. A., An. Inst. Cienc. del Mar y Limnol. Population structure of the Dab, Limanda limanda (L.) in Isle of Man waters. Univ. Nal. Autón. México, 1981d. 15 (1) 1988. STEVEN, G. A., J. Mar. Biol. Ass. Bottom fauna and the foof of fishes. UK (N.S.), 1930. 677-706. 16: TOOD, R. A. Mar. Biol. Ass. , Second report on the food of fishes (North Sea 1904-1905). UK 1907 50-163. pt. 1:
|

