|
SEDIMENTARY PYRITE IN A TROPICAL LAGOON:
APPLICATION OF THE DIAGENESIS EQUATION FOR CALCULATING SEDIMENTATION RATE, MEAN
SALINITY AND RATE OF PYRITIZATION
Trabajo recibido el 5 de marzo de 1986
y acpetado para su publicación el 10 de septiembre de
1986
Laurence D. Mee
Instituto de Ciencias del Mar y Limnología,
Estación Mazatlán. Universidad Nacional Autoónoma de
México.
Pedro Ortega Romero
Centro de Investigaciones Cientificas y
Tecnologicas de la Universidad Sonora (CICTUS, Hermosillo, Sonora,
México. Contribución 525 del Instituto de Ciencias del Mar y
Limnología,
UNAM
Se
estudió la distribución vertical detallada de los componentes
principales del ciclo de azufre en sedimentos obtenidos de cuatro puntos en la
laguna "El Verde", Sinaloa, México, en dos estaciones del año.
Estas fueron, la estación de lluvias, cuando la laguna (S= 4.1%)
está comunicada con el mar y es receptora de descargas fluviales, y la
estación de sequía, cuando la laguna permanece aislada (S =
29.7%). Los datos obtenidos de un núcleo de 70 cm permitieron la
aplicación de un modelo matemático para describir la
distribución de pirita (un componente que no mostró marcadas
variaciones estacionales) empleando la ecuación general de
diagénesis. El modelo permitió la estimación de la tasa
media de sedimentación para el sistema (0.6 cm/año) y la
reconstrucción del perfil medio de sulfato intersticial en el
núcleo. Esto, a la vez, hizo posible calcular la salinidad del sistema
promediada sobre varias décadas (15.4%). El modelo generó
información sobre la tasa de formación de pirita en los
sedimentos sub-superficialcs. Se demostró la importancia de la capa
biomezclada de sedimentos en la producción inicial de
pirita.
The
detailed vertical distributions of the principie components of the sulphur
cycle were measured at four points in the El Verde lagoon, Sinaloa,
México, at a period when the lagoon was an insolated water-body (S =
29.7%, dry season) and another when it was comniunicated with the sea and river
discharges (S = 4.1%, rainy season). Data from a 70 cm long corc permitted
modelling of the pyrite distribution (wich did not show seasonal variations)
using the general equation for diagenesis. The model resulted in the obtention
of an cstimate of 0.6 cm/yr for the mcan sedimcntation ratc in the systcm and
the reconstruction of the average interstital sulphate profile in the core.
Ibis, in turn, made it possible to estimate the long-term mean for lagoon
salinity as 15.4%. The model provided information on the rate of pyritization
in the sub-surface sediments. The importance of new pyrite production in the
bioturbatcd layer was
demonstrated.
The systematic study of tropical coastal lagoons began only some 20 years ago and as a consequence, our knowledge of the recent history of these water bodies is very limited. In the case of México, with more than 120 coastal lagoons (Lankford, 1977), the data base for making environmental management decisions for individual lagoons is often deficient or non-existent. Since these lagoons show very large seasonal and inter-annual variability in their hydrography, biology and chemistry (Mee, 1978) and are transient geological features (Lankford, 1977), the problem of cost and time effecctive environmental monitoring is a very complex one. Even such basic information as the annual mean salinity, the sedimentation rate and the long-term stability of primary organic production, may be very difficult to obtain. The paleo-oceanographic information contained in the sediment record gives valuable clues to the solution of these problems. Studies of authigenic minerals in temperate arcas, and particularly those of sulphide minerals started by Emery and Rittenberg (1952) and Berner (1964), have provided the basis for modellíng the genesis and distribution of these important sedimentary components in relation to the general conditions under which they were formed. The present work was aimed at exploring the application of these models to examine the pyrite distribution in lagoon sediments and thus obtain some basic information on the recent history of the lagoon itself. STUDY AREAThe El Verde lagoon (Fig. 1) is a small (0.5 km² ) barred inner shelf lagoon (Lankford, 1977) situated at 23°51' N and 106°34' W, just north of Mazatlán on the Mexican Pacific coast. It consists of a 1m deep and 7 km long narrow channel parallel to the coast and fringed by mangroves. It is feed during the rainy season (July-October) by the river Quelite. During these months, the lagoon bar opens to the sea but remains closed the other 8 months of the year. Previus studies of El Verde have examined the seasonal variations in dissolved nutrients, salinity and temperature (Galindo,1981), primary production and detrital supply (Flores-Verdugo, 1985) and the biochemistry of some dissolved organic components (González-Farias, 1985). 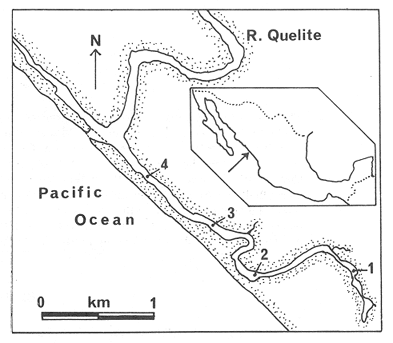 Figure 1. Map showing sampling points in the Verde lagoon. METHODSVery short (10 cm long, 5 cm diameter) cores were taken by hand from 4 stations in the south-eastern branch of the lagoon (Fig. 1) in June and October 1981. A larger core (70 cm long, 6.6 cm diameter) was taken at station 2 in October 1982. Bottom water samples were also collected at cach core station. Cores were immediately sealed in plastic bags and stored at 5 °C for transport and analysís. In the laboratory the short cores were extruded and cut into 1 cm sections. The long core was cut into 2 cm sections. Each section was then sub-divided into three parts, to be analyzed respectively for (i), water content, organic material and trace metals, (ii), interstitial water components and (iii), sulphur cycle components. Water content was determined by weight loss after drying to constant weight at 65ºC. Organic carbon was estimated in the dried sediments by the method of Walkley and Black (1934), as modifed by Loring and Rantala (1977), in which the organic carbon is oxidised by dichromate/sulphuric acid and then back titrated with ferrous ammonium sulphate. Interferences are controlled using phosphoric acid and sodium fluoride. Easily leachable trace metals were extracted for 6 hours from the dried sediments using 2M nitric acid at 100 °C in teflon beakers. The metals extracted were analyzed with an Atomic Absorption Spectrophotometer. Interstitial water was extracted from the second part of each section by centrifugation and decantation. Small aliquots of the water were employed for sulphíde, suP phate and pH measurements. Sulphide was determined using a silver/silver sulphide electrode (Berner, 1963; Midgley and Torrance, 1968). Sulphate was measured (following barium sulphate precípitation) using the indirect EDTA/Eríochrome Black-T titration method of Howarth (1978). The pH was determined using a glass electrode calibrated with NBS buffers. Sulphur cycle components were analyzed using a purpose-built anaerobic digestion system similar to those employed by Kaplanet al.(1963) and Jorgensen and Fenchel (1974). The system is designed in such a way as to enable it to be continually purged with nitrogcn gas and allows all reagents to be nitrogen purged and anaerobically stored. The sample is placed in a three necked, stoppered 250 ml round bottom Pyrex "Quickfit" flask on a magnetic stirrer/heater. Nitrogen is injected through a narrow-bore tygon tube inserted through the first stopper and led to the bottom of the flask. Reagents (4M HCI and distilled water) may be added via two inlets through the second stopper. A tube through the third stopper leads to two traps (in series) containing 2% cadmium chloride solution for collecting hydrogen sulphide. Two to three grams (wct weight) of sediment are weighed into the flask, air is removed, hydrochloric acid is added and the system, is heated to 35 °C with stirring. The sulphide liberated is trapped in the cadmium chloride solution to be late r released with acid and determined iodometrically (Orland, 1965). The acid in the flask is decanted off and the sediment is washed twice with distilled water. Sulphate is determined in the combined extract and wash water bv the method of Howarth (1978). Pyrite, together with any elemental sulphur present, is determined in the remaining sediment (dried or partially dried by centrifugation) using a brominc/carbon tetrachloride/nitric acid digestion and oxidation (in the presence of aluminium powder) described by Kolthoff and Sandell (1952) and analysis of the sulphate produced as described above. RESULTSOnly the results revelant to the development of the mathematical model will be presented and discussed here. The full data set will be discussed in a later paper. Average water column salinities for the June (1981), October (1981) and October (1982) studies were 29.7 %, 4.1 % and 22.8 % respectively. The difference between the two October figures illustrates the general inestability of the system during the rainy season where occasional heavy rainfall in the catchment area of the river Quelite will rapidly lower the lagoon salinity. The surface sediment organic carbon concentration increased from a value of 2.5% (of dry weight) at station 4 (close to the highest tidal energy areas of the lagoon, near the bar opening and river mouth) to about 7% at station 1 (furthest from the bar and with the densest mangrove vegetation). Sediments were generally silty with some sand fractions at stations 1 and 2. Comparison of interstital water sulphate analyses with those of total sulphatc revealed that virtually all sedimentary sulphate was present in the dissolved form. Interstitial sulphate concentrations near the sediment surface were similar to those of the overlying lagoonal water column. Sulphate concentrations generally exhibited only very small gradients in the surface 6-10 cm (bioturbated) layer of sediments and then decreased exponentially. The reverse of this was observed with the case of sulphide which showed a low but uniform concentration in the bioturbated layer and then increased, almost exponentially to some fairly constant value by 40 cm depth. The thickness of the bioturbated layer (as observed in sulphate and sulphide profiles) varied from 4 cm (June, 1981) to 10 cm (October, 1981), perhaps reflecting the seasonal differences in the available hydro-mechanical energy in the water column (causing physical mixing), rather than biological mixing. Surface sediments sulphate concentrations varied from 22.8 µg-at S-S04²-CM -³ (station 4, June 1981) to 4.1 µg-at S-S04 ²- cm-³ (station 1, October 1981). Surface sulphide concentrations varied from 10.75 µg-at S-S²- cm-³ (station 1, June, 1981) to undetectable (station 3 and 4, October, 1981). In the case of the long core (Octi/ober, 1982), surface sulphate and sulphide concentrations were 16.1 µg-at S-SO²- cm-³ and 7.1 µg-at S-SO²- cm-³ respectively, with corresponding values of 5.25 µg-at S-S04 ²- cm-³ and 16.8 µg-at S-S04 ²- cm-³ at 67 cm depth. These values are similar to the previous measurements in coastal and estuarine sediments summarised by Goldhaber and Kaplan (1974). Pyrite typically represented 50-85% of the total sulphur in surface sediments and 87% in deeper sediments (October, 1982, 67cm depth). This latter figure corresponds to a sedimentary pyrite component of 1.4% of the total sediment dry weight. The depth distribution of the three principal componcnts of total sulphur at station 2 are illustrated in figure 2. The sulphide fraction includes the casily decomposed non-stoichiometric metal sulphides such as mackinawite (FcSo.9). It can be appreciated from the figure (June and Octobcr, 1981 data) that whilst large seasonal variations in sulphate and sulphide are observed, the pyrite concentration remains virtually unaltered. The depth profiles of organic carbon and easily leachable (non pyrite) iron are illustrated in figure 3 for the June station 2 data. Iron represents about 2.3% (dry weight) of the total sediments. The degree of pyritization of the sediments (measured by the concentration of Pyrite iron/(Pyrite iron + easily leachable iron)) varies from 11-26 %, a fraction almost identical to that observed in Connecticut (USA) coastal sediments by Berner (1970) and showing that the iron concentration is not a factor limiting pyrite production. 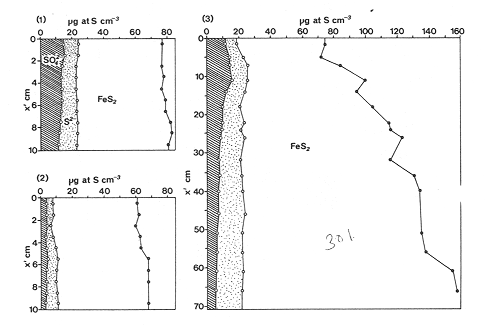 Figure 2. The cumulative distribution of the three main components of the Sulphur cycle in lagoon sediments. The three cores were obtaincd from station 2 during the June 1981 survey (core l), the October 1981 survey (core 2) and the October 1982 survey (core 3). Sediment depths for this, and subsequent figures will be given in terms of x', the depth below the sediment/water column interface. 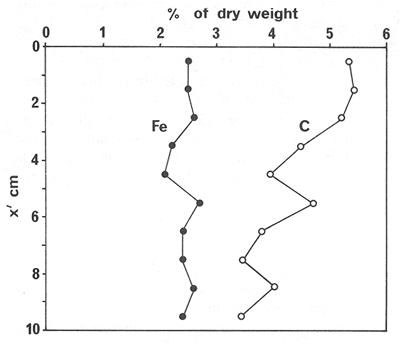 Figure 3. The distributions of organic carbon (C) and easily leachable iron (Fe) in the core taken station 2 in June 1981. Values are expressed as % of dry weight. DISCUSIONThe formation of authigenic sulphate minerals in sediments of recent deposition has been described mathematically, using the general diagenesis equation, by Berner (1964, 1971, 1974, 1980), Assuming that: (1) The descomposition of organic matter during suP phate reduction follows first-order kinectics; (2) The chemical reaction affecting dissolved sulphate in the interstitial water is bacterially mediated reduction; (3) Sulphate adsorption is minimal; (4) Compaction and porosity gradients can be ignored; (5) diffusion is controlled by molecular processes only; (6) The diagenetic processes are steady-state, then it can be shown (Berner, 1974) that the rate of sulphate reduction 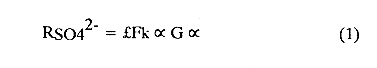 where £ is the stoichiometric coeficient for the number of ions of sulphate reduced per atom of carbon oxidized (generally 0.5), G α is the concentration of metabolisable carbon compunds (α - carbon, which only represents a small fraction of the total organic carbon in the sediments), k α is the decay constant of α -carbon and F is a factor for normalizing the results in convenient units (F =Þs(1-ø)/ø, where ø is the porosity and Þs the mean sediment density). Using the assumptions (1) - (6), Berner (1980) showed how the general equation for diagenesis could be símplified and integrated between carefully defined boundary conditions to generate equations for the interstitial sulphate concentration (c) and the metabolizable organic carbon concentration (G) at any x below the bioturbated sediment layer (the upper boundary of the model): 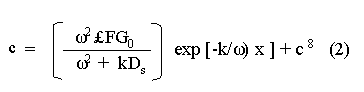 and 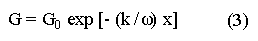 Here ω is the sedimentation rate, Go is the concentration of metabolizable organic carbon at the bottom of the bioturbated layer, Ds is the molecular diffusion coefficient for sulphate in the sediments, k is the k α previously defined and C ∞ is the sulphate concentration oberved where all the α-carbon has been used up and is the lower boundary of the model. This model has been successfully employed to describe the interstitial sulphate distribution in a wide variety of environments, ranging from the deep-sea to high sedimentation rate areas of the continental shelf (Berner, 1980) and the first-order kinetics hypothesis for α-carbon descomposition was recently tested experimentally (Jorgensen 1978a, b; Westrich and Berner, 1984). The assumptions of the model are largely met in the present case except for that of a steady-state. This is clearly apparent from figure 2 where a wide range of seasonal variations are observed in the interstitial sulphate profiles. If the sulphate distribution is to be modelled, a steady-state assumption cannot be applied. Within the analytical errors of the current data set, there were practically no seasonal variations for the pyrite (+ elemental sulphur) distribution in the sediments. The question therefore arises as to whether pyrite may be regarded as a steady-state component of lagoon sediments. In order to resolve this matter it is thus necessary to study the experimental evidence and mechanism for pyrite formation in the lagoon. It is important to point out that "steady-state" here refers only to the geochemical conditions over the period for which the model is valid (ie.x'/ω where x' is a value of x where c––>c∞). Lagoons cannot be regarded as geologically "steady-state" environments and are, of course, transient features (see Mee, 1978). The sedimentary sulphur cycle is fully described by Goldhaber and Kaplan (1974). In order to form pyrite, dissolved iron is required together with reduced forms of sulphur (HS- and S²-) and small quantites of native sulphur which is formed by near-surface sulphide oxidation. The bacterial reduction of sulphate is fuelled by α carbon (Berner, 1974) and initially the entire process may occur in reducing microcosms around macroscopic organic detrial particles trapped whithin the upper few centimetres of the sediment, whithin the otherwise oxidized bioturbated layer (Emery and Rittenberg, 1952). The full importance of this near-surface pyrite production has only recently been appreciated by modelling (Berner, 1980) or by field and laboratory uptake experiments with35S labelled inorganic sulphur compunds (Howarth, 1979; Howarth and Jorgensen, 1984). The present data set gives clear evidence for nearsurface pyrite production. Sulphate and pyrite data were differentiated with respect to depth for two of the cores with high total organic carbon concentrations. The depth distributions of d[SO4 ²-]/dx and d[pyrite]/dx were smoothed using a three point running mean and plotted as a function of depth below the sediment surface (Fig. 4). From the figure, a maximum in the rate of sulphate reduction occurs simultaneously with an apparent peak of pyrite production at about 2-3cm depth. The depth of these maxima would be related to the time lapsed after deposition by dx = wdt, assuming no bioturbatrion. This assumption is not valid however as the distribution of d[/SO4 ²-])/dx changes seasonally and no permanent pyrite maximum is observed in the sediments. The pyrite concentration in the El Verde lagoon was generally found to be fairly uniform down to about 6cm. The sediments down to this depth were probably both bioturbated, and perhaps more importantly, physically mixed by stirring during summer storms. 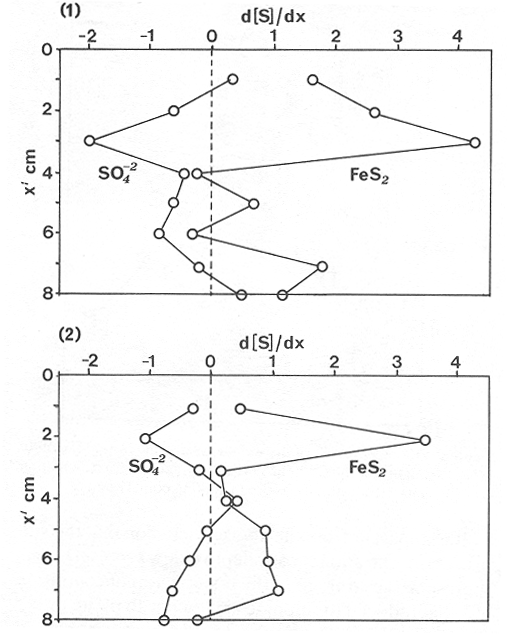 Figure 4. profiles of d [S]/dx for sulphate and pyrite obtained from cores taken during the June 1981 survey at station 1 (diagram 1) and station 2 (diagram 2). Units for d[S]/dx are *g at SO4 2- cm -3. Below the turbated zone, seasonal fluetuations would be expected in the sulphate profile down to a depth of x = √2Dst where Ds is the diffusion coefficient for sulphate and t is the frecuency of the cyclic change (in the present case tmax is one year). The variation in sulphate concentration at any one depth does not imply a variability in the rate of pyrite generation, except at very low concentrations of sulphate, as the rate-limiting process is α-carbon oxidation (an excess of sulphate is usually present). This was recently demostrated experimentally by Boudreau and Westrich (1984). The clear relationship between surface pyrite and total organic carbon can be observed in figure 5 (line AB). Following burial of any one sediment layer, new pyrite is formed as the organic carbon is used up (direction CD in figure 5). 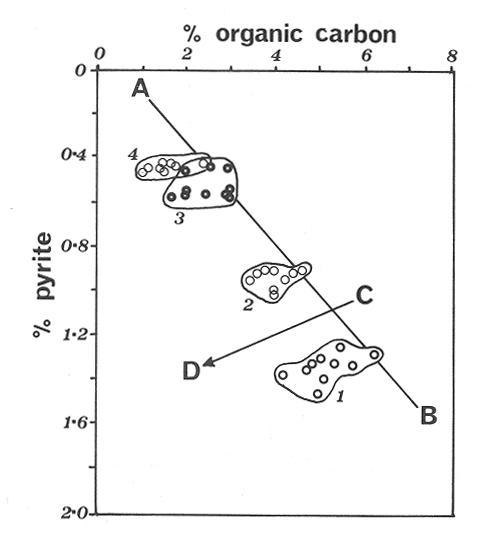 Figure 5. Plot of pyrite (% of dry weight) vs organic carbon (% of dry weight) for the October 1981 survey. See text for explanation. Data are clustered by core (sampling point) number. It is apparent from the above discussion that the sulphate concentration for any depth below x = √2Dst will represent the average surface sulphate concentration less that reduced to sulphide and thence to pyrite byα- organic carbon. The pyrite profile however, should provide a permanent record of the average sulphate concentration for any given depth, and at the sulphate concentrations observed in the present data set the pyrite formation should be a steady-state process. This conclusion allows equations (1)-(3) to be applied in the following manner (adapted from Berner, 1980). The sum of the authgenic sulphur minerals (ΣS, principally pyrite with a small amount of elemental sulphur) produced to any depth of undisturbed sediments will be 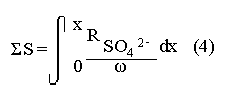 Substituting (1) in (3) gives 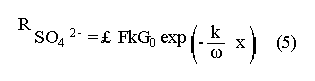 Sustituting this result in (4) and integrating: 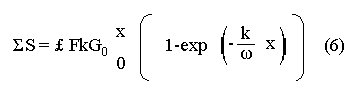 This result implies that the concentratíon of pyrite should increase with depth with the same exponential gradient as equation (2). The data obtained from the 70 cm core at station 2 (see Fig. 2) was used to study the application of this model. The exponential curve was fitted to the data by a linear least-squares procedure (given that a plot of [pyrite + Sº] versus [1-exp(-k/ax)] should be a straight line with zero intercept). The only unknown fitting parameter is (k/w) which is estimated by the upper boundary of the curve which is simply the pyrite concentration at the bottom of the bioturbated layer. This value is subtracted from all pyrite concentrations used in the model (but must be taken into aecount when plotting the data). The result of this fitting procedure (shown on Fig. 6) was the equation: ΣS= 121.7 [1 - exp (-0.024 x )] (7) which described the data very well (r² = 0.96). From (7) and (6), it is apparent that £FG0 = 121.7. As £ for this reaction is 0.5 and F was calculated (given that ρ = 3 and φ = 0.77) as 0.9, then G0 (the initial quantity of metabolizable carbon) would be 2.70 x 10-4 moles cm-³ which is about 0.3% of the sediment dry weight. Toth and Lerman (1977) have shown, for a wide range of sediments, that: k = Aω² (8) 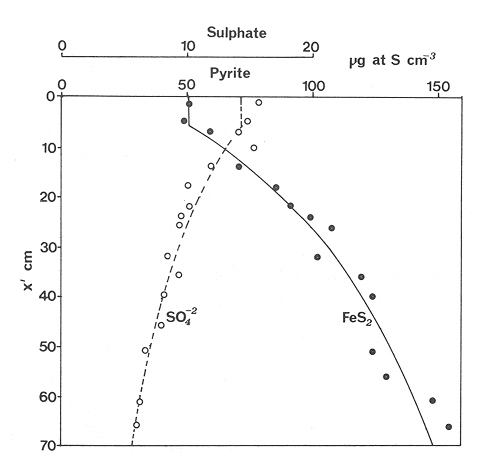 Figure 6. Application of the model to the pyrite data from the core obtained at station 2 in October 1982 survey. The solid line represents the "best-fit" model. The broken line shows the average interstitial suíphate concentration if scawater were penetrate the system for any length of time. where A is an experimentally derived constant of 0.04 cm-² yr. From (8) and (7) k/ω = Aω = 0.024. The sedimentation rate for the El Verde lagoon was thus estimated as 0.6 cm yr-¹. This is quite similar to that measured by the ²¹º Pb technique (0.93 cm yr-¹) by Paez-Osuna and Mandelli (1985) in the Mitla lagoon, Guerrero, México. If the pyrite distribution can be modelled, then it is reasonable to suppose that a mean annual sulphate profile can be regenerated using equation (2). The only additional term in this equation is the sulphate diffusion coefficient in sediments (Ds). This may be estimated from the diffusion coefficient of this ion in seawater (D) and the tortuosity (Ø) (see Berner, 1980): DS = d/ز (9) McDuff and Ellis (1979) have shown that ز = ØØ-n(10) where a good working value for n would be 1.8. Substituting the previously derived values of ø in (10) and (9) and utilizing the tabulated value of D = 9.8 x10-6/cm² sec given by Berner (1980) yields a value of Ds of 8x10 -6 cm-² sec-¹. By subtituting this value in equation (2), the average sulphate profile for station 2 would be described by: 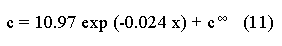 The c ∞ value in this equation may be found by numerically fitting it to the lower three sulphate data points (well below √2Dst). Application of this procedure yields a coo of 3.25. The reconstructed sulphate curve is shown in figure 6. It can be seen that this curve fits the field data rathrc well, though at depths less than √2Dst (22.5cm) the fit may only be considered as fortuitous as the sulphate concentrations vary seasonally. The model gives a mean sulphate concentration in the bioturbated layer (co) of 14.22µ moles cm-³ (wet sediment), or in terms of the concentration in the interstitial water, 12.44 mm. The field observations showed that sulphate concentration in the interstitial water of the bioturbated layer closely followed that of the overlaying water column. Thus this mean value should also reflect the mean water column sulphate concentration. In the absence of sulphate reduction, the water column chlorinity/sulphate ratio should be constant (Marcet's Principle) at 0.1400 g kg-¹ C1 %-¹ (Riley and Chester, 1971). The mean chlorinity for about the past 50 years must thus have been approximately 8.5%, representing a salinity of 15.4%, just slightly lower than that measured for 1979 (19.7%) by Galindo (1981). Galindo noted however that 1979 was a year of exceptionally low river runoff and the lagoon salinity was probably somewhat higher than average. In figure 7, the sulphate profile at three different times of the year is illustrated using the avaible field data and showing the calculated mean profile. The good fit of the model to the pyrite data has another paleoecological implication. From figure 5 and equations (1)and (2) it can be seen that the rate of pyrite formation is closely related to the quantity of organic carbon initially deposited in the lagoon. Small year to year variations would be smoothed out in the bioturbated zone (ten year's deposition). Longer-term variations however would completely alter the form of the pyrite profile. This implies that supply of organic material to the lagoon has not suffered any major changes over the past 100 years. Flores-Verdugo (1985) has recently shown that almost all of the organic material deposited in the lagoon is produced within it, principally from phytopiankton and bordering mangrove communities and it may be concluded that the production of these communities is remarkably stable on a long-term basis. lf it is assumed that all of the sulphate reduced eventually becomes pyrite, then the mean rate of pyritization for any given sediment layer may be calculated (µg-at S cm-³ time-¹) by integrating equation (5) over fixed limits and then diving by the thickness of the layer chosen: 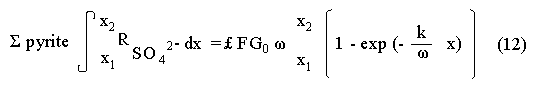 The application of this equation is illustrated in figure 8. lf it is assumed that most of the pyrite in the bioturbated layer was locally produced, the observed rate of pyritization for this layer is the product of the mean pyrite concentration within it and the edimentation rate. This result is shown on figure 8 and is almost three times that predicted by the model. This high surface pyritization rate was observed in other studies and it has been suggested (Westrich and Berner, 1984) that a more reactive form of α-carbon exist near the sediment surface but wich also follows first-order decomposition kinetics. 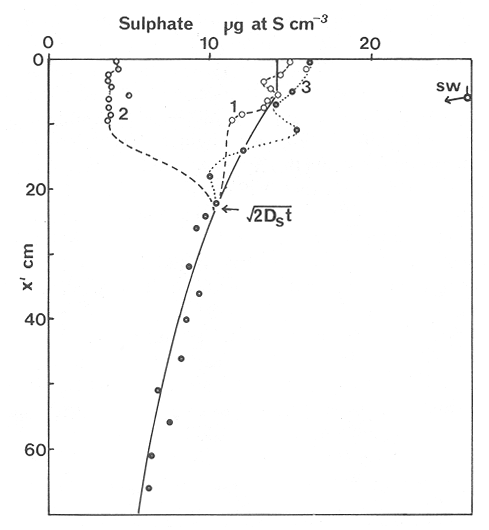 Figure 7. Reconstruction of the seasonal variations in the interstitial sulphate profile at station 2. Cores are numbered as in figure 2. The solid line is the modelled mean for the system and broken lines connect field data. "SW" indicates the interstitial sulphate concentration if seawater were to penetrate the system for any length of time. A summary of the results from the present study is presented in Table 1 and compared with three earlier studies organic-rich sediments. Present results are of a similar order to the earlier work. It is however, interesting to note that despite the large quantity of α -organic carbon (Go) avaible in the El Verde, the decay constant (Ks) is quite low. A recent study of the organic matter in the El Verde by Gonzalez-Farias (1985) has demonstrated the presence of large quantites of tannins and demostrated their antibiotic or bacteriostatic behaviour. Just how much this affects the rate of decomposition of organic matter by sulphate reducing bacteria remains to be seen.  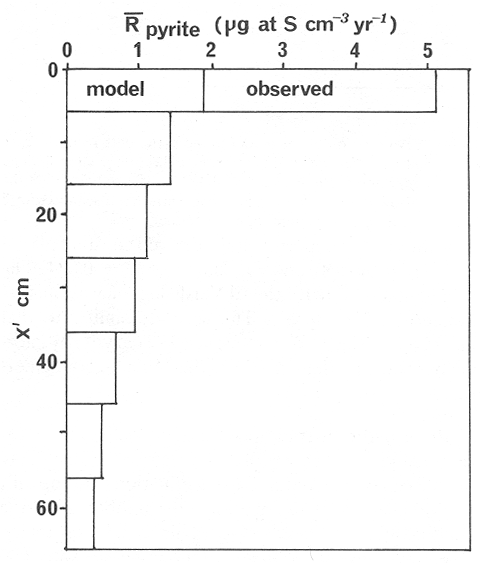 Figure 8. The mean rate of pyrite production in core 3, obtained from the model. The rate (R) is calculated for 10 cm sections of the core downwards from the base of the bioturbated layer. The rate is also presented for the 6 cm bioturbated layer and compared with the observed mean pyrite production rate. See text for details. ConclusionesThe distribution of authigenic pyrite, an essentially steady-state component of coastal lagoon sediments, provides valuable information on the biogeochemistry of the lagoon system as a whole. Mathematical modelling of the pyrite distribution and application of the general equation for diagenesis allows an estimate to be made of the sedimentation rate. It also permits reconstruction of the mean interstitial sulphate profile from which information such as the mean lagoon salinity may be obtained. The present data base compares well with other (temperate water) studies but in order for this approach to be widely adopted, rescarch should be conducted in other tropical areas. 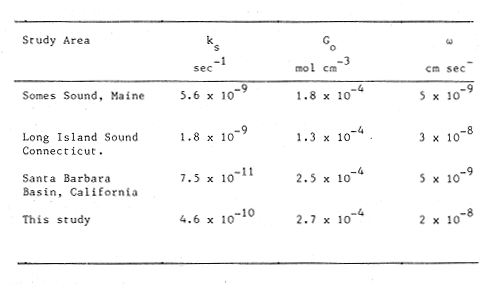 TABLE 1 COMPARISON OF PARAMETERS ESTIMATED FROM THE PRESENT ESTUDY WITH THOSE OBTAINED IN OTHER COASTAL AREAS AND SUMMARISED BY BERNER (1980) AgradecimientosThe field and laboratory work was carried out at the Mazatlán station of the Instituto de Ciencias del Mar y Limnología, UNAM. The assistance of the head and staff of this station are gratefully acknowledged. The present text was prepared during the senior author's leave at the International Laboratory of Marine Radioactivity, Monaco. The helpful comments and suggestions of the staff were greatly appreciated. LITERATURABERNER, R.A.Geochim. Cosmochim. ActaElectrode studies of hydrogen sulphide in marine sediments.1963.563-565.27 BERNER, R.A. Geochim. Cosmochim. ActaAn idealized model of dissolved sulphate distribution in recent sediments.1964.1497-1503.28: BERNER, R.A.Sedimentary pyrite formation.Amer. J. Sci.1970.1-23.268: BERNER, R.A. Principles of chemical Sedimentology.McGraw-HillNew York.1971.240 p. BERNER, R.A. The sea Kinetic models for the early diagenesis of nitrogen, sulphur, phosphorus and silicon in anoxic marine sediments. In: Goldberg, E.D. (Ed.)Wiley-InterscienceNew York.1974.427-450.Vol. 5. BERNER, R.A. Early Diagenesis - A theoretical Approach.Princeton University PressNew York.1980.241 p. BOUDREAU, B.P. and J.T. WESTRICH Geochim. Cosmochim. ActaThe dependance of bacterial sulphate reduction on sulphate concentration in marine sediments.1984.2503-2516.48 EMERY, K.O. and S.C. RITTENBERGBull. Amer. Petr. Geol.Early diagenesis of california basin sediments in relation to origin of oil.1952.735-806.36 FLORES-VERDUGO, F. Tesis de Doctorado (Especialidad Oceanografía Biológica)Aporte de materia orgánica por los principales productores primarios a un ecosistenla lagunar-estuarino de boca efímera.Universidad Nacional Autónoma de México, Colegio de Ciencias y Humanidades, Unidad Académica de los Ciclos Profesional y de Postgrado.1985. GALINDO, J.G. Tesis de Maestría (Especialidad Oceanografía Química)Estudio preliminar sobre la productividad primaria y la dinámica de los nutrientes en el Estero El Verde, Mazatlán, Sin., México.Universidad Nacional Autónoma de México, Colegio de Ciencias y Humanidades, Unidad Académica de los Ciclos Profesional y de Postgrado.1981. GOLDHABER, M.B. and I.R. KAPLAN The Sea The sulfur cycle. In: Goldberg, E.D., (Ed.)Wiley-InterscineceNew York.1974.569-655.Vol. 5. GONZÁLEZ-FARÍASTesis de doctorado (Especialidad Oceanografía Biológica)Importancia ecológica de la materia orgánica y su biodegradación en el estero El Verde, Sinaloa, México. Universidad Nacional Autónoma de México, Colegio de Ciencias y Humanidades, Unidad Académica de los Ciclos Profesional y de Postgrado.1985. HOWARTH, R.W. Limnol. Oceanogr. A rapid and precise method for determining sulfate in seawater, estuarine waters and sediment pore waters.1978.1066-1069.23 HOWARTH, R.W. Science Pyrite: Its rapid formation in a salt marsh and its importance in ecosystem metabolism.1979.49-51.203: HOWARTH, P-W. and B.B. JØRGENSEN Geochim. Cosmochim. ActaFormation of 35 S-labelled elemental sulphur and pyrite in coastal marine sediments (Limfjorden and Kysing Fjord, Denmark) during short-term 35 SO4 ²- reduction measurements.1984.1807-1819.48: JØRGENSEN, B.B. J. Geomicrobiol.A comparison of methods for he quantifiation of bacterial sulfate reduction in coastal marine sediments. 1. Measurement with radiotracer techniques.1978a.11-27.1: JØRGENSEN, B.B. J. Geomicrobiol.A comparison of methods for he quantifiation of bacterial sulfate reduction in coastal marine sediments. 2. Calculations from mathematical models.1978a.29-51.1: JØRGENSEN, B.B. and T. FRENCHEL The sulfur cycle of a marine sediment model system. Mar. Biol.1974.189-201.24: KAPLAN, I.R., K.O. EMERY and S.C. RITTENBERG. Geochim. Cosmochim. Acta. The distribution and isotopic abundance of sulphur in recent marine sediments off southern California.1963297-331.27: KOLTHOFF, I.M. and E.B. SANDELL A textbook of Quantitative Inorganic Analisys Mc Millan 3ra. ed.New York1952.759 p. LANKFORD, R.R.Estuarine Processes. Coastal lagoons of Mexico, their origen and classification.In: Wiley, M. (Ed.). Academic Press.London.1977.441-490. LORING, D.H. and R.T. RANTALA Fisheries and marine Service Technical Report 700 Geochemical analyses of marine sediments and suspended particulate matter.Canada1977.58 p McDUFF, R.E. and R.A. ELLISDetermining diffusion coefficients in marine sediments: A laboratory study of the validity of resistivity techniques.Amer. J. Sci.1979.666-675.279: MEE, L.D Chemical Oceanography. Coastal lagoons. In: Riley, J.P. and R. Chester (Eds.) Academic PressLondon.1978.441-490 p. MIDGLEY, D. and K. TORRANCEPotentiometric Water Analisys.Wiley-InterscienceNew York.1978.409 p. Standard Methods for the Examination of water and WastewasterORLAND, II.P. (Ed.)American Public Health Association Inc. 12th. edn. New York1965.769 p. PÁEZ-OSUNA, F. and MANDELLI, E.F. Estuar. Coast. Shelf Sci.210pb in a tropical coastal lagoon sediment core.1985.367-374.20: RILEY, J.P. and R. CHESTERIntroduction to Marine ChemistryAcademic PressLondon.1971.465 p. TORH, DJ. and A. LERMAN Organic matter reactivity and sedimentation rates in the ocean. Amer. J. Sci.1977.265-285.26: WALKELEY, A. and I.A. BLACK An examination of the degthareff method for determining soil organic matter and proposed modification of the chromic acid titration method.Soil Sci.1934.27-38.27: WESTRICH, J.T. and R.A. BERNER Limnol. Oceanogr. The role sedimentary organic matter in bacterial sulphate reduction: The G model tested1984.236-249./29:
|

