|
ECOLOGY, BIOLOGY AND POPULATION DYNAMICS
OF ARCHOSARGUS RHOMBOIDALIS (PISCES, SPARIDAE) IN A TROPICAL COASTAL LAGOON
SYSTEM, SOUTHERN GULF OF MEXICO
Paper presented preliminarily in the
Fourth Congress of European Ichthyologists, Hamburg West Germany, 20-24 Sep.
1982. Recibido el 25 de junio de 1984 y aceptado para su publicación el
20 de octubre de 1984.
PIERRE CHAVANCE
Station Marine d'Endoume, Rue Batterie des Lions 13007
Marseille (France).
ALEJANDRO YÁÑEZ-ARANCIBIA
DOMINGO FLORES-HERNÁNDEZ
ANA LAURA LARA-DOMÍNGUEZ
FELIPE AMEZCUA LINARES
Universidad Nacional Autónoma de
México, Instituto de Ciencias del Mar y Limnología, México
04510, D. F., Apartado Postal 70305, México. Contribution 301 from the
Instituto de Ciencias del Mar y Limnología, UNAM.
El sargo, Archosargus rhomboidalis, es una especie
no capturada comercialmente que se encuentra en la Laguna de Términos
durante todo el año en áreas donde predomina la influencia marina
y están bien representados los pastos marinos. La evolución
estacional de la población está fuertemente influenciada por el
reclutamiento que ocurre en la época de lluvias de julio a octubre. Se
encuentran dos principales áreas de crianza sobre praderas de pastos
marinos en el litoral interno de la laguna. Conforme crecen, los peces se
mueven a la vecindad de la Boca de Puerto Real que es el área de
reproducción. Cuatro etapas, desde carnívoros estrictos a
omnívoros, se encuentra en la dieta del sargo desde las larvas al adulto
y se relacionan con el tamaño de la boca, cambios en la dentición
y habitats. La madurez sexual tiene lugar de enero hasta julio aunque es
más intensa de enero a mayo, en la época de secas. Las hembras
representan el 60 % de la población adulta; y no se observa
inversión sexual. La primera maduración ocurre cuando los peces
alcanzan 80 mm de longitud estándar (LS) i. e., los 5
meses de edad. Los cambios en la alimentación y en la actividad sexual
están relacionados en los cambios de la condición del pez. El
crecimiento es estudiado por distribución de frecuencia de longitudes
mensuales y marcas de crecimiento diarias en otolitos; las constantes del
modelo de crecimiento de von Bertalanffy son: K = 0. 125, LSoo = 200 mm y
t0 0.815 meses. El sargo tiene 2 años de
longevidad. La tasa de mortalidad natural es Z 1.98 (año)
correspondiendo al 18% de pérdida de número por año. La
biomasa del sargo en la laguna el evaluada en 2,500 tons métricas dando
una captura potencial de 2,646 tons.
The seabream,
Archosargus rhomboidalis, is a commercially unfished specie
found at Terminos Lagoon which occurs all year round where marine influence
predominantes and seagrass beds are widely present. Seasonal evolution of
population is heavily influenced by recruitment that takes place from july
until October, the rainy season. Two main nursery grounds are found above
seagrass beds in the inner part of the Lagoon: As they grow, fishes move to the
Puerto Real inlet vicinity, which is the area of reproduction. Four stages,
ranging from stricty carnivorous to omnivorous, were found in the seabream.
diet from larvae to adult and were related to size mouth, changing dentition
and habitat. Sexual maturation takes places from january until july although it
is more intense from january until May, the dry season. Females represented 60
% of the adult population; no sexual inversion is observed. First maturation
occurs when when fish reaches 80 mm standard length, i.e.
when 5 months old. Changes in alimentation and sexual activity are related to
changes in fish condition. Growth is studied from monthly length frequency
distribution and daily growth marks on otoliths; von Bertalanffy growth model
constants are: K = 0. 125, SLoo = 200 mm and t 0= 0.815
month. The seabream has a 2 year longevity. The instantaneous rate of natural
mortality is Z = 1.98 (year) corresponding to a 18 % loss in numbers per year.
Seabream biomass in the Lagoon is evaluated at 2,500 metric tons giving a
potential yleld of 2,464 tons.
The coastal lagoons and estuaries of Mexico are the object of extensive programs of investigation in order to evaluate their potential biological resources and to obtain information on production mechanisms in tropical coastal systems. One of these ecosystems is the Terminos Lagoon in the Southern Gulf of Mexico, situated near Campeche Sound, where important fishery (shrimp) and industrial (petroleum) activity is taking place. Archosargus rhomboidalis, the seabream is one of the most common fish species in the study area. Yáñez-Arancibia et al. (1980, 1983b) report this species as the second in number (16.3 % of captures) among 83 species found in the Puerto Real Inlet area, and the fourth (8 %) among 116 found in the inner part of the Lagoon. In relation to weight, the seabream is the first (19 % of catches weight) in the inlet area and the second (19 % ) in the Lagoon. Seabream is not exploited at present in the lagoon and constitutes a potential resurce that must be taken into consideration. The seabream along with the pinfish, Lagodon rhomboides, and the sheephead Archosargus probatocephalus, are the sparids most commonly found in the coastal and estuarine systems of the Gulf of Mexico. Whereas the sheephead can be collected with the seabream of the pinfish all around the Gulf, the last two are exceptionally reported in the same areas as indicated in figure 1. According to the zoogeographical analysis of Briggs (1974), pinfish is mainly reported in the northern part of the Gulf corresponding to the Carolinian Province (tempera te) . Seabream is reported in the Caribbean Province and the West Indian Province (tropical). Only Low (1973 In Houde, 1976) reports both species in Biscayne Bay, Florida, where it has been previously observed that tropical fishes prevalent in summer are partially replaced in winter by temperate species (de Sylva, 1976). Briggs reports the seabream from New jersey, on the United States Atlantic coast, to Rio de Janeiro, Brazil. This species actually appears more frequent-in the tropical part of this range, and is replaced by the pinfish in the temperate part. There are very few publications dealing with Archosargus rhomboidalis and most of them, are experimental studies on eggs and larvae. Houde (1974, 1975, 1978) studied under laboratory conditions the influence of temperate, delayed feeding, critical food concentrations and stocking density upon larval growth and survival. Houde and Potthoff (1976) descrived egg and larval development, and Stepien (1976) studies the feeding rate, food selection and daily ration of larvae in the laboratory. Very recently, Chavance et al. (1983) studied eggs and larvae of the seabream at Terminos Lagoon. Regarding adult fish, we may cite Vaughan (1978) who investigates adult food habits in nature and compares growth on plant and animal food in the laboratory. The objetive of this study is to analyze the seasonal and spatial distribution of the seabream, in Terminos Lagoon, its feeding, reproduction and to estimate some parameters of its population such as biomass, condition factors, growth, mortality and potential yield. STUDY AREASouthern Gulf of Mexico has a warm humid climate with three climatic seasons. From June until the end of September, there are almost daily afternoon and evening showers. October to january is the season of "nortes" or winter storms. February through May is the dry season. Precipitation ranges between 1,200 and 2,000 mm per year with a mean of 1,681 mm. 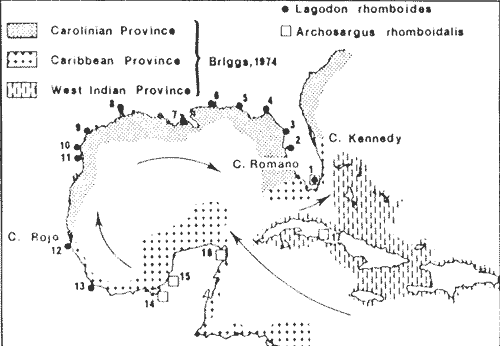 Fig. 1. Reports of Lagodon rhomboides and Archosargus rhomboidalis in the Gulf of Mexico and adjacent areas. General water circulation and zoogeographical provinces according to Briggs (1974) are also indicated. 1, Low in Houde and Potthoff (1976). 2, Lindall et al. (1973). 3, Carr and Adams (1973). 4, Livingston (1976), Subrahmanyam and Drake (1975). 5, Ogren and Bruscher (1977). 6, Swingle (1971). 7, Sabins and Truesdale (1974), Fox and Mock (1968). 8, Holt (1976). 9, Simmons and Hoese (1959). 10, Gunther (1958). 11, Hellier (1962). 12, Reséndez (1970). 13, Reséndez (1973). 14, Yáñez-Arancibia et al. (1980). 15, Yáñez-Arancibia et al. (1982) 16, Reséndez (1975). 17, Gonzáles-Sanson et al. (1978). Terminos Lagoon (Fig. 2) is a large (1,567 Km2 ) and shallow (average depth 3.5 m) coastal lagoon connected with the sea by two inlets. Prevailing easterly winds, longshore currents and river discharge cause a strong net flow into the eastern. inlet (Puerto Real) and out from the western inlet (El Carmen)- Tidal range is 0.3 to 0.7 m. Water temperature ranges from 22 to 31°C. Salinity ranges from 12 to 300/00 in the open lagoon. Total river discharge averages 6x1O9 m3 year-1. Puerto Real Inlet has a broad delta of calcareous sediment owing to the net import of water and sediment from the sea, and presents clear waters. Temperature ranges from 24 to 34°C and salinity from 22 to 390/00. El Carmen inlet has temperatures ranging from 22 to 31°C and salinities from 4 to 330/00. Sediments are silty clays and waters are highly turbid. Ecological descriptions of Terminos Lagoon, Puerto Real inlet, Carmen inlet and the adjacent fluvial-lagoon systems are found elsewhere (Bravo Núñez and Yáñez-Arancibia, 1979; Amezcua Linares and YáñezArancibia, 1980; Yáñez-Arancibia and Day, 1985 a; Graham et al., 1981; and other references quoted in those papers). 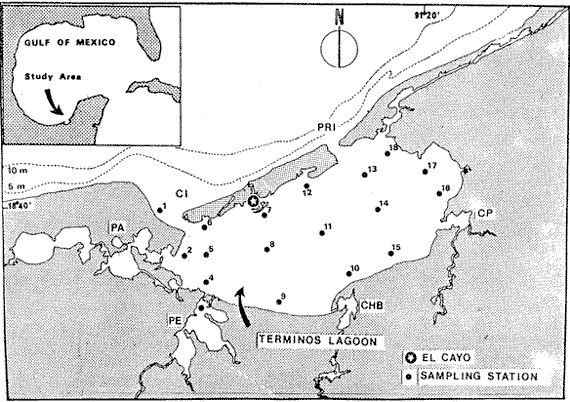 Fig. 2. The study area. Geographical localization of the Terminos Lagoon and sampling stations situation. CI = Carmen inlet, PRI Puerto Real inlet. Fluvial-lagoon systems, PA = Pom-Atasta, PE = Palizada del Este, CHB = Chumpan-Balchacah, CP Candelaria-Panlau. METHODSFifteen cruises were made from February 1980 until April 1981 at monthly intervals. Eighteen sampling stations were located in Terminos Lagoon at approximately 10 kms intervals where depths ranged from 1.5 to 3.5 m (Fig. 2). Fishes were collected during daylight hours with a 4.9 m (16 foot) otter-traw1 with a 314 inch mesh. Tows were made during 12 minutes at 2 or 2.5 knots. A 2,000 m2, sample area was considered for density and biomass computation since the traw1 was observed by Loesch et al. (1972) to sweep an area 2.5 m in width. Traw1 efficieney, determined by the same authors, is 25 % for Atlantic croaker (Micropogon undulatus) and only 6% for spot (Leiostomus xanthurus). We utilized a 20 % value for the seabream. At the same time, plankton samples were collected for ichthyoplankton studies. The corresponding results concerning seabream eggs and larvae are reported by Chavance et al. (1983). Temperature, salinity and transpareney were recorded at each station and the results are reported by Yáñez-Arancibia et al. (1983 c).
In the laboratory, seabreams were measured and
weighed. When not specified in the text, lengths are expressed in standard
lenght
Total length (Ltot) is related to standard length (SL) as follows:
Ltot = 2.27 SL - 0.02 with length in mm. r = 0.997; N = 902
Food habits of 20 fishes were investigated by the gravimetric method (Windell and Bowen 1978). Individuals from 30 to 150 mm proceed from collections made from May until july 1980 at station 7. Specimens larger than 160 mm proceed from Station 13 made on September 1980. Digestive Tracts were immediately removed after collection and preserved in 10 % sea waterbuffered formalin. In the laboratory, stomach contents were sorted in 6 categories: Al, algae; Pv, vascular plants; Mol, molluscs; Cr, crustaceans; De, unidentified detritus; Oa, other animals. Each category of food was dried at 95°C for 24 hours, and weighed. Results are then expressed in terms of total stomach weight percentages. Basic data were processed using subprograms of the S.P.S.S. (Social Package for the Social Science, version 7) (Nie et al. 1975) on a computer Burroughs 6700. Subprograms FREQUENCY, CROSSTABS, REGRESSION, SCATTERGRAM, and AGGREGATE were mostly used. Data were processed by monthly cruise and also globally. Length-weight relationships were computed by regression and are expressed as the following: P = bLa in which: P = total or eviscerated fish weight in g; L = standard length in mm; b = mean condition coefficient; a = allometric coefficient. The seasonal evolution of a and b were analyzed. Relative condition coefficents were also studied. Fulton's index was utilized for eviscerated (Kl) as well as total (K2) weight: K1 = 105 P1/L³ and K2 = 105 P2/L³ in which: Pl = eviscerated weight in g; P2 total weight in g; L is defined above. Le Cren's index (1951) was also computed: KN1 = 105 P1/b1La1 and KN2 = 10 5P2/bLa2 in which: Pl, P2 and L are defined above; b = mean condition factor for eviscerated (bl) and total weights (b2); a = allometric coefficient for eviscerated (al) and total weights (a2). Fulton and Le Cren's coefficients were very redundant since calculated allometric coefficients were fairly elose to 3 and thus did not justify Le Cren's correction for non-isometric growth. For this reason, only Fulton's coefficient will be discussed in this study. The relative proportion of viscera was analyzed using the following index computed from Kl and K2: 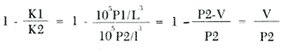 in which: V= weight of viscera in g; Other parameters are defined above. Growth was studied by the Petersen method, but this method is not fully efficient in tropical areas. Further discussion may be found in Fagade (1974). Daily growth marks on otoliths constitute a new tool for age identifications particulary useful in tropical areas where classical methods are poor1y realiable, These marks, which constitute pairs of adjacent bands, one light and one dark of approximately 10 μm, were first described by Pannella (1971) on adult fishes. Seabream otoliths were processed as follows: otoliths were washed with water and then were secured laterally to a glass mieroscope slide with Canadian balsam. The exposed side of the otolith is then ground by hand against a mixture of carborundum (n° 400) until the otolith nueleus is observable. A similar operation is done on the other side before proceeding to a 30 second immersion in 1 % HCI. Four successive and independent readings were made for each otolith and those giving more than a 10% variation between counts were eliminated. A von Bertalanffy growth model was fitted to the average ring count and the fish standard length by determining first the better asymptotic length (SL 00 ). using Beverton's method (In: Ricker 1975 P. 225). We have: SL = SL α -(SL α -Sl0) exp (- Kt) or SL = SL α [1- exp (-K (t-t0))] in which SL = length at age t; SL α= asymptotie length; Sl0 = length at age t = 0; t0 = age when length equals 0; K = growth coefficient; t = age Mortality was determined from the rate of decline of total catches in relation to length. An exponential model was fitted to the data. Afterwards, the instantaneous rate of mortality in terms of length obtained, was multiplied by the better linear rate of growth in order to obtain the instantaneous rate of mortality in terms of ages. Fish abundance in relation to length is subject to errors as the escape of small fishes through net meshes, non availability or gear avoidance of larger individuals. Therefore, only fully recruited length classes were considered for mortafity studies. RESULTS AND DISCUSSION
SPATIO-TEMPORAL DISTRIBUTIONThe seabream is collected all year round in the Lagoon (Fig. 3, Table 1); the average density fluctuates from 0.00719 ind. per m2 in February 1981 to 0.04588 in September 1980. This index varies almost steadily from low values in February to higher ones in September. Biomass varies equally from 0.060029 g per M2 in February 1980 to 0.552529 g per m2 in September 1980. However, biomass remains almost constant during the first seven months of sampling and then shows 3 peaks respectively in September, November and january. These high values result from uncommon catches at stations 13 and 18 also related to high values of the average length index, indicating that those catches are mainly made of large individuals. It is likely that large specimens are not satisfactorily collected by our catching procedure. Except for these 3 particular months, it is to be noticed (Fig. 3), that the average length index diminishes regularly starting from February and reaches very low values from July until October and then increases until the beginning of the year. This index reflecting the relative importance of juveniles in our catches makes us conclude that recruitment to the population and to our fishing area is maximum from july until October corresponding to the rainy season. Density is also high at that time. 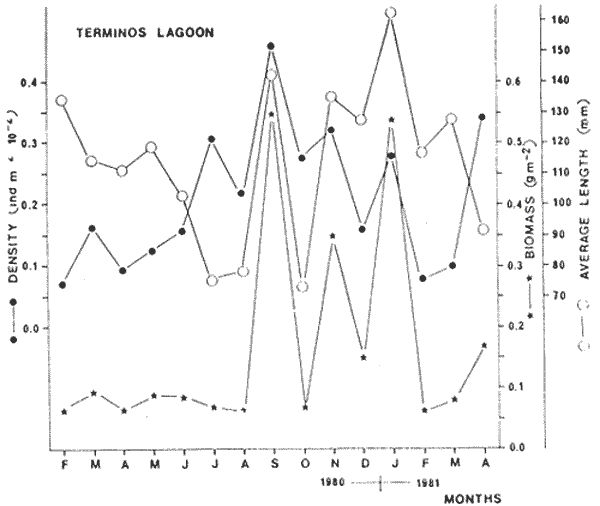 Fig. 3. Seasonal evolution of density, biomass and average length for the seabream.
TABLE 1 Seabream population characteristics are therefore high by affected by the recruitment process that is taking place yearly during the rainy season. Figure 4, indicates the number of fishes caught at each sampling station all over the sampling period as well as the corresponding length frequency distribution. Archosargus rhomboidalis is mainly distributed on the inner side of Carmen Island and in the northeastern region of the Lagoon; seabream is uncommon in the central part and very rare in the southern and southeastern parts of the Lagoon. This pattern of distribution elearly coincides with that of seagrass beds. Three areas of major ocurrence can be distinguised. They are those corresponding to stations 7, 16, 13 and 18. The station 7 area (El Cayo) is characterized by and elevated frequeney of occurrence (86.7 %), a low average length(89.44 mm) although a large range of length is represented (Cv = 30.91 %). Station 16 presents similar characteristics but with less numerous captures. On the other hand, station 13 is very distinct since at this station the frequency of occurrence is low (60 %), the average length is high (144.46 mm) and length coefficient of variation is low (10. 59 % ). Station 18 is very similar to station 13, however, juvenile fishes may be collected at station 18, although unfrequently.
Fig. 4 Consequently, there exists a well defined pattern of fish distribution according to length or age in the Lagoon. Station 7 is localized in the main nursery area, station 16 in a secondary one and station 18 in an oceasional one. Stations 13 and 18 are situated in areas where large or old individuals are mostly found. Therefore, we must assume that fishes migrate from the nursery areas to areas near the Puerto Real Inlet. This assumption is confirmed by the demographic structure of station 12 situated between stations 7 and 13, because it is elearly intermediate between those of stations 7 and 13. Seabream spawning area and larval drift studied by Chavance et al (1983) enable us to complete the migration pattern. These authors show that spawning occurs mainly in the Puerto Real Inlet vicinity and that larvae are then transported by currents in two directions, towards El Cayo and towards the eastern part of the Lagoon, i. e. towards the direction of the main as well as the secondary nursery area identified in this study. The migration pattern is therefore coherent: it consists of a) a denatant movement of sexual products and larvae from the Puerto Real Inlet to the nursery areas situated above seagrass beds and b) a contranatant movement to the Puerto Real Inlet vicinity, when fishes reach approximately 100 mm. FOOD HABITSSome observations on teeth development and digestive tract morphology have been done. Seabream dentition is characterized by incisor (i) and molariform teeth (m), the number of which changes in the following manner, as the fish grows.  Molariform teeth are nonfuctional in 40 mm long fishes and become firm in 70 mm long ones. Digestive tract is characterized by a large intestinal part that always constitutes nearly 90 % of' the total digestive tract length and averagas twice the fish standard length. Seven digestive caeca are attached to the stomach near the pyloric region. Stomach contents show significant variation according to fish length, indicating ontogenetic changes in diet. Three stages can be distinguished (Fig. 5): – Young fishes (30-80 mm) eat mostly crustaceans (35 % of dry weight) among which predominate gammaridean amphipods. Copepods, ostracods and young specimens of Brachyouridae ar also found in their sto machs. They eventually eat polychaetes. Plants are only slight1y represented (Al = 2.7%, Pv = 0.5%) and include fragments of Thalassia testudinum and unidentified filamentous green algae. 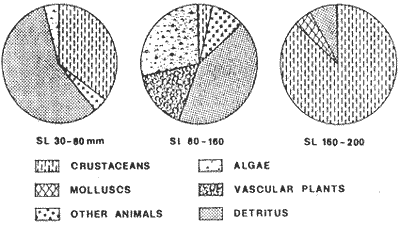 Fig. 5. Seabrearn stomach contents in relation to fish length. – Fishes from 80 to 160 mm feed most y on plants (Al + Pv = 45 %) such as unicellular algae, filamentous algae and Thalassia testudinum. Animal food items are not abundant (Mol + Cr + Oa = 5 %) but are diversified since we find bryozoans, colonial tunicates, poly and oligochaetes worms and sometimes fish eggs and small fishes. Crustaceans are poorly represented and are gammaridean amphipods, ostracods and a few tanaids. Montascs (0.3 %) include remains of gastropod and bivalve shells. – Large fishes (160-200 mm) eat mostly animals (Mol + Cr + Oa = 91 %) among which predominate undiversified crustaceans. A fish stomach was completely filled with a juvenile paeneid shrimp, 50 mm long. In general, tanaids are the more abundant food item. Molluscs, mostly bivalves, are more numerous than in other length groups. The proportion of detritus appears very variable, even within an alimentary stage. However, it always remains low among large individuals, whereas it can reach 90 % of stomach contents in other length groups. Food habits of seabream have been studied by Randall (1967) and with more precision by Vaughan (1978) in Biscayne Bay, Florida. The latter observes significant changes in feeding aceording to fish length; crustaceans are the major food items for fishes from 41 to 79 mm Fork Length; larger fishes (80-199 mm F. L.) eat mostly plants and very large ones (> 200 mm F. L.), mostly molluscs. This pattern is very similar to our observations; the only remarkable difference is that molluscs were never dominant in our fish stomachs; very large fishes eating mostly tanaids. The origin of this difference is likely to be found in a distinct availability of these two groups in Terminos Lagoon and Biscayne Bay. Stepien (1976) studies in laboratory conditions the food selection of seabream larvae and shows that larvae eat copepod naup1ii, copepodites and adult copepods; the size of ingested preys being related to mouth size. Chavance et al. (1983) observed that larvae up to 8 mm, in natural conditions (Terminos Lagoon), eat essentially copepods and oceasionally algae; larger larvae migrate to the bottom vicinity and feed on amphipods, isopods and young decapods. In fact, mouth morphological. characteristics (size and dentition) make larvae and juveniles, up to 70 or 80 mm, obliged carnivores. Afterwards, as molariform teeth become solid and functional, fishes (80-160 mm) feed mostly on seagrass beds. Ingestion of plant material is then important and the animal food component is diversified from leaves, stems and emerging part of rhizomes epifauna. Although fishes smaller than 80 mm may be found in seagrass beds, it is probable that they live in more coastal waters and move progressively to deeper zones where seagrass beds are well developed. Catch curves (Fig. 12) confirm this point of view since they show that fishes up to 110 mm are unadequately collected. Evidently, these fishes tend to be distributed in areas where our sampling was deficient, i. e.. in very shallow coastal waters where depths are too low for a traw1 net to operate. Furthermore, this was confirmed by large captures of juveniles smaller than 70 mm that we made with a beach seine in the nursery area of El Cayo, where depths range around 1 m. Very large fishes ( > 160 mm) are mostly found in tidal canals near Puerto Real Inlet as can be seen in figure 4. Quantitative changes in diet are therefore related to fish movement in the Lagoon as synthetized in Table 2.  TABLE 2 CHANGES IN SEABREAM DIET AND HABITAT ACCORDING TO FISH LENGTH Sparids are generally found to be primarily carnivorous, feeding mainly on benthic fauna like crustaceans, polychaetes and gastropods. However, some species, mainly among the coastal subfamily of Diplodinae, tend to ingest great quantities of plant material especially vascular plants such as Thalassia, Diplanthera, Syringodium. Such is the case of Lagodon rhomboides, Archosargus probatocephalus, Diplodus holbrooki (Darnell, 1958; Springer and Woodburn, 1960; Odum and Heald, 1972; Carr and Adams, 1973) and Archosargus rhomboidalis (Randall, 1967; Vaughan, 1978). Vaughan demonstrates experimentally that the seabream cannot grow normally when feed only on plants indicating that during the 80-160 mm stage, seagrass epifauna plays an important role in natural alimentation. However, growth is seemingly affected when fishes change from a strict carnivorous to an omnivorous diet when length averages 80 mm, since the length-weight relationship is elearly modified, as will be detailed later. Fishes less than 80 mm have a higher allometric coefficient than fishes longer than 80 mm. When seabreams return to a strict carnivorous diet at round 160 mm, no modification could be observed in the length-weight relationship, probably because data are few and also because growth is then greatly reduced. Seabream food habits elearly demonstrate that the trophic level concept may be inoperative for a single species during its whole lifetime. Seabream strategy, and certainly that of many estuarine species, is characterized by large feeding potentialities, interhabitat movement and the ability to consume detritus whose importance in estuarine food webs has already been emphasized by Odum and Heald (1975). REPRODUCTIONThe numbers of male and female collected were grouped by month, and data reveal females to be more abundant in the catch than males (Table 3). Generally, sex ratios differ significantly from the expected 1: 1 ratio (Female: Male), and actually range around 1.5: 1. The November ratio remains significantly distinct from 1.5: 1, and perhaps refleets monosexual schooling. Sex ratios, grouped into 10 mm standard length intervals, also have significant departures from expected 1: 1 ratio (Table 4). In general, females predominate and the 1.5: 1 ratio can be accepted for all length classes, except the 80 mm length class for which data are certainly unreliable, since it is easier to identify a young female than a young male during the beginning of the reproduction period. 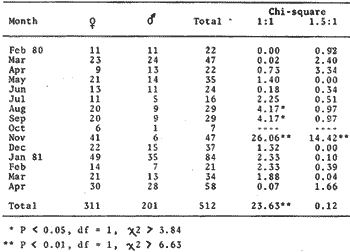 TABLE 3 NUMBER OF FEMALES AND MALES COLLECTED ACCORDING TO SAMPLING PERIOD AND CHI-SQUARE VALUES(x2) FOR A 1:1 OR 1.5: 1 RATIO (FEMALE/MALE). 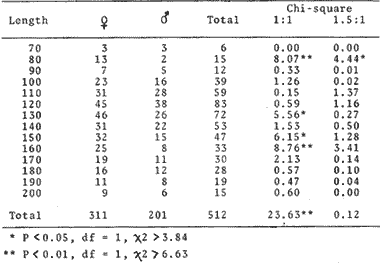 TABLE 4 NUMBER OF FEMALES AND MALES ACCORDING TO STANDARD LENGHT CLASSES AND CHI-SQUARE VALUES (X2) FOR A 1:1 AND 1.5:1 RATIO (FEMALE/MALE). Hermaphroditism is common among sparids (d'Ancona, 1950, 1956; Atz, 1964) this family provides a progression from species with protandrous or protogynous hermaphrodites through rudimentary hermaphrodites to species that are structural and fuetional gonochorists. Archosargus rhomboidalis seemingly pertains to the lat ter type, however our macroscopic sex examinations may have failed to reveal a rudimentary hermaphroditism like the one found by d'Ancona (1956 In Atz, 1964) in a very closely related species, Archosargus probatocephalus, the sheephead. Nevertheless, oocytes and nests of spermatocytes next to one another were never observed among the 512 individuals we identified sexually. Figure 6, shows that the majority of females are maturing from january until june and that maturation is more intense from january until May, the dry season. Maturation is slight from August until December. Chavance et al. (1983) collected seabream eggs in Terminos Lagoon from january until july but found that spawning is more intense from january until April. Therefore, according to both types of data, spawning occurs during the dry season. 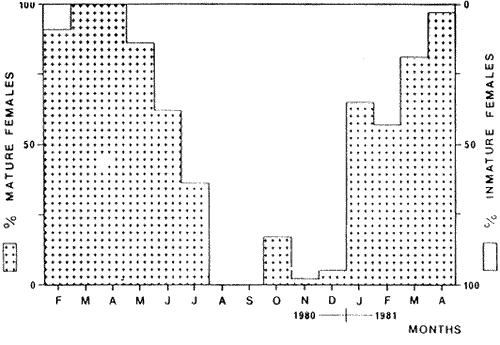 Fig. 6. Monthly percentages of mature (stages II, III IV) and inmature (stage I) seabrearn females. The smallest female collected that was undergoing a maturation process measured 84 mm. Data on maturation stage in relation to fish length were analyzed (Fig. 7) in order to determine at what length 50 % of females are expected to mature during the reproduction period; it appears that length at first maturity is 80 mm. This fish length corresponds to a 5 month old fish which will be discussed later. Therefore, the seabream is able to reproduce during the first spawning season after birth. Seabream fecundity has already been studied by Chavance et al. (1983) and the relative fecundity was estimated at 484, 720 oocytes per kg. Adult females can spawn various times during the spawning season. 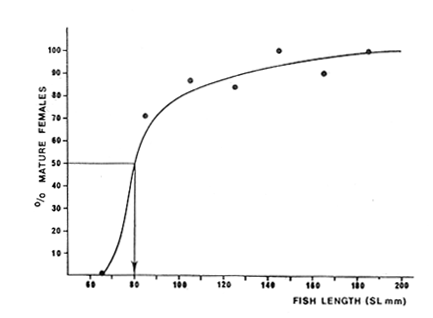 Fig. 7. Seabream length at first maturity. Proportion of maturing females during the spawning periods (February 1980 - June 1980 and February 1981 - April 1981) in relation to fish length. Pelvic fin colouration appears related to fish sex. They are totally or partially dark in males and orangecolored in females. Among 100 fishes tested, the sex of only two was misidentified, using the pelvic fin colouration criterion. LENGTH-WEIGHT RELATIONSHIPS AND CONDITION FACTORSRelationships between fish length (standard length, SL in mm) and fish weight (Total weight, P2 in g and eviscerated weight, P1 in g) are calculated for all fishes during the sampling period. We have: P2 = 2.7310-5 SL 3.07195 r = 0.99602 N = 901 and P1 = 2.57 10 -5 SL 3.06113 r = 0.99546 N = 912 Separating fishes by sex, we have for eviscerated weights: Females P1 = 3.65 10 -5 SL 2.98698 r = 0.98995 N = 311 Males P1 = 4.49 10 -5 SL 2.93882 r = 0.98956 N = 201 Length-weight relationships are very similar for males and females. On the other hand, it appears from data analysis that two equations is a better representation of the length-weight relationships for all lengths, one for lengths lower than 80 mm and one for lenghts higher than 80 mm. We have: Standar length < 80 mm: P1 = 0.92 10 -5 SL 3.31491 (1) r = 0.97570 N = 252 Standar leigth > 80 mm: P1 = 5.41 10 -5 SL 2.90823 r = 0.99003 (2) N = 660 It is important to put emphasis on the fact that 80 mm is a significant length in the seabream life cycle since it is the length at first maturity and also the length when fishes change from shallow coastal habitats where they are strictly carnivorous to seagrass meadows where they are omnivorous. Coefficients of length-weight relationships (P = bLa), computed monthly for all fishes without length consideration, show significant seasonal fluctuations as indicated in figure 8 top. The allometric coefficient (a) and the mean condition factor (b) vary inversely, indicating that when mean condition is low, weight gain is high. Such is the case of fishes smaller than 80 mm as illustrated by equations (1) and (2). The allome tric coefficient (a) increases from the beginning of the year until August and then decreases, the lowest values being observed in March 1980 and March 1981. The mean condition coefficient (b) fluctuates with inverse characteristics. From the fact that important collections of juveniles occur from july until October and that juveniles are characterized by high values of a and low values of b, we deduce that seasonal evolution of coefficients a and b reflects on the relative proportion of juvenile fishes in the population, that is to say the recruitment process. 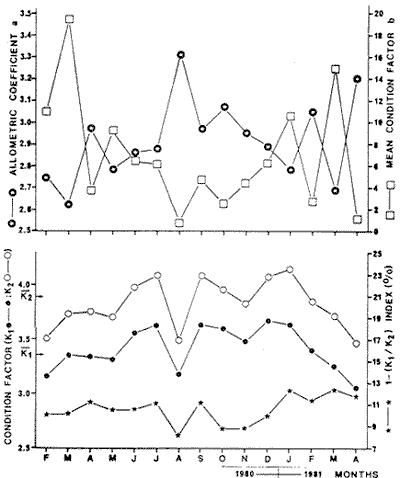 Fig. 8. Seasonal evolution of: Top-allometric coeflicient and Mean condition factor. Bottom-relative condition coefficient and visceral index. Figure 9, shows that fish relative condition coefficients are lower in small fishes than in large one. K1 and K 2 increase rapidly with length and reach their maximum values around 90 mm. This is evidently related to the difference in allometry that characterizes fishes smaller from those larger than 80 mm. K1, decreases slowly when length increases, whereas K 2 remains almost constant indicating that the proportion of viscera is increasing from 150 to 230 mm as illustrated by index 1-K1/K2 . The seasonal evolution of coefficients (Fig. 8 bottom) shows that K1, and K 2 present values above the mean from june until February and below the mean from March until May. The August value disagrees with this general tendency and it probably results from important captures of small fishes that are characterized by low relative conditions or from deficient data collection. Percentages of viscera show less clear fluctuations; however, from February until july 1980 and from january until April 1981, they are elevated and are evidently related to the reproduction period. September 1980 gives and abnormally high value when reproduction is over, which is probably due to the exceptional capture made in this particular month of large individuals (see spatio-temporal distribution) which are characterized by elevated percentages of viscera as discussed previously. 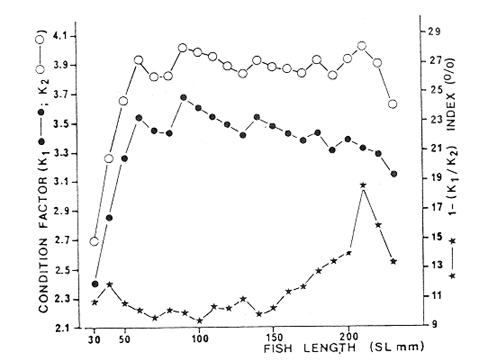 Fig. 9. Relationship between relative condition factor, Visceral index and fish length. To conclude, we may consider the following explanation valid: during the reproductive period (january until july) percentage of viscera is high because gonads are maturing. Fish relative conditions is low because of the maturation effort; fishes are getting thinner. The allometric coefficient is then lower than 3 because the population is mainly constituted by adults, fishes being able to reproduce during the first spawning season after birth. Outside the reproduction period, the percentage of viscera is low and the relative conditions of fishes is then high because fishes are fattening. The allometric coefficient is then higher than 3 since a major part of the population is constituted by juvenile fishes. BIOMASS ESTIMATIONSeabream average biomass in the Lagoon, estimated monthly, presents important fluctuations as shown in figure 3 and Table 1. Three peaks may be observed, respectively in September 1980, November 1980 and january 1981. As discussed in the spatio-temporal distribution analysis, it is likely that these variations result from inadequate sampling. Average biomass varies from 0.060029 g/m² in February 1981 to 0.552529 g/m² in September 1981. The highest value observed is 8.7775 at station 13 in September 1980. For the whole sampling period, average biomass is 0.166186 g/m². It is likely that large specimens are undersampled, thus our direct biomass estimate must be in general, undervalued. Therefore the better estimate must be found between the average biomass for the whole sampling period and the highest monthly biomass estimate, i.e. 0.1661860.55252 g/m². Using a lagoon area of 1,567. 106 m2 and considering that net efficiency is 20 %, seabream biomass in the Lagoon ranges between 1,300 and 4,325 metric tons. Chavance et al. (1983), obtained an adult biomass estimate of 2,751 tons from ichthyoplankton studies in the same area and during the same period. It is known that these studies give estimates within a range of variations defined by 50 % below the estimated value and 100 % above, that is 1 376.5 502 tons. Therefore, it appears that both methods give similar estimates, and that seabream biomass in the Terminos Lagoon averages 2 500 tons. Seabream is not distributed evenly all over the lagoon (Fig. 4), and occupies almost 1/3 of the entire area; therefore we will consider that seabream reaches a biomass equal to 48 kg/ha in those areas where it is distributed, i.e in the north and northeastern parts of the Lagoon. GROWTHResults obtained through the Petersen method are shown on figure 10. Interpretation of mode displacement along the time axis appears to be very subjective in many occasions, specially when modes are made of very few individuals. These results constitutive then only of elements for comparison with those of a more reliable method. However, some preliminary observations may by made: – April 1980, july 1980 and April 1981 are characterized by the occurrence of a modal length class at 40 mm, indicating the arrival of young fishes in our sampling area and their first capture by our net juveniles are actually caught with consistency from April until October but these 2 anual modes indicate the occurrence of 2 major arrivals; one proceeding from individuals born during the beginning of the spawning period (january) and the other from individuals born later (from April-May). – In a general manner, 3 or 4 modal length classes are observable monthly, which would correspond to 2 year classes; each one constituted by early born individuals and those born later. Longevity is therefore around 2 years. - The largest modal length class observed is 200 mm, it constitutes a first approximation of the asymptotie length. – Individuals measuring 40 mm when captured for the first time in April 1980, measure 150 mm in February, 1981, giving an average growth rate of 11 mm per month. 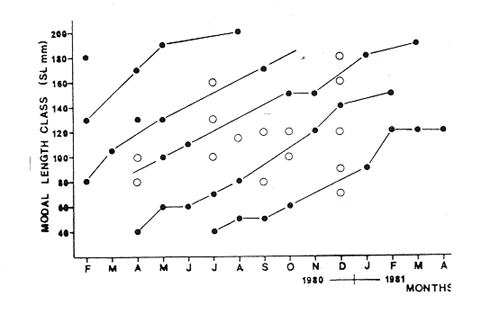 Fig. 10. Seasonal evolution of modal length classes. Open symbols correspond to small numbers of fish. Daily marks on otoliths were easy to read on young fishes but presented increasing difficulties for older ones, resulting in important variability in successive readings. Therefore, only fishes smaller than 130 mm were aged by this method. A von Bertalanffy growth model was calculated using the Beverton's method (In Ricker, 1975) which is shown in figure 11 as well as the exponential larval growth model calculated by Chavance et al. (1983) in the same area. An equivalent and commonly used form of the v.B. growth model is: SL = 200 [1-exp (-0.0041 (t-24.8),] SL in mm, age in days. These equations can be modified according to units required for comparisons. With regard to time units, we have:  The estimated asymptotie length of 200 mm is a reasonable figure since among 1,070 fishes examined, only 11 were larger than this size including 6 that pertained to the 210 mm length class. The largest individual collected had a 230 mm length. This is acceptable since SLoo must be regarded as an average. 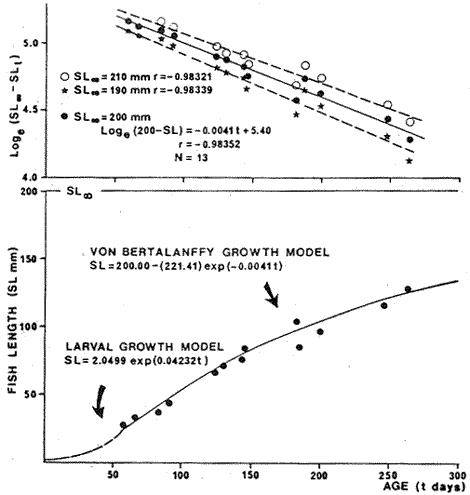 Fig. 11. Seabream growth as determined from daily growth in crements on otoliths. Top -determination of the better asymptotic length by Beverton's method (see text). Bottom -von Bertalanffy gorwth model fitted to data and larval growth model determined by Chavance et al. (1983). A linear model can also describe growth satisfactorilly; the regression model is: SL = 0.484 t + 2.990 With SL in mm and age in days; or SL = 1.471 t + 0.299 With SL in em and age in months r = 0.97862 N = 13 From this model, we deduce that individuals grow 1.5 em every month which is only a little more than what has been indicated by the Petersen method. According to Caldwell (1957) and Hansen (1969), the seabream grows faster than the pinfish, Lagodon rhomboides which grows approximately 6 mm per month during summer; growth being greatly reduced in winter for this temperate species. Zuñiga (1967) gives for Boops boops (Sparidae) on the Spanish east coast, a von Bertalanffy model with K = 0.171, L α = 352 mm and tΣ = - 2.064 year. Arias (1980) for Sparus auratus (Sparidae) on the Spanish west coast indicates K = 0. 130, L α = 845.3 mm and to = - 1.586 year. K-values higher than 1, on a yearly basis, are rare in fish literature according to Beverton and Holt (1959) and Beverton (1963), which are seemingly typical of small pelagic (Engraulids, in particular) and tropical species. Taylor (1958) defines life span as the time required to attain 95 % of the asymptotie length, A (0.95). Considering the von Bertalanffy growth model fitted to our data, we have A (0.95) = 756 days, i.e. almost 2 year. Preliminary results from the Petersen method are in close agreement with this assumption. Population is thus constituted of 2 year-classes with 2 subgroups according to birth date. Lengths of oneyear-old fishes range between 120 and 150 mm. When 2 years old, fishes average 190 mm. The von Bertalanffy growth model for weight is deduced from the length-weight relationship which is: W = 319.8 [1- exp (-0.0041 (t-24.8))] 3.07195 With weigth in g and age in days. A 6 months, 1 year, and 2 years old fish weight, respectively, 32.7, 133.3 and 268.3 g. MORTALITYCatch curves based on length frequencies for males, females and for all fishes collected are shown in figure 12. Three remarks may be made: – The 30 and 40 mm length classes are not adequately represented which is mainly because corresponding fishes are able to escape through net meshes. – From 50 to 110 mm length frequencies are also abnormally low; escapes being discarded, we must consider that these fishes are not fully available. This is in agreement with the gut contents analysis since it appeared that fishes from 8 to 80 mm feed on particular preys and are very likely distributed in shallow coastal waters. 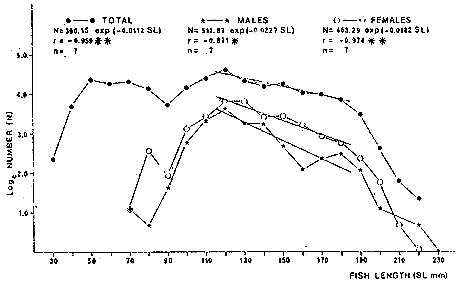 Fig. 12. Seabream catch curves and exponential mortality models fitted. – Catch curves become concave for lengths greaterthan 190 mm. Ricker (1975: p. 48) stresses that an increase in natural mortality among old fishes (senility) is commonly found in unexploited populations. Although the case might be that inadequate sampling and net avoidance of larger fishes in particular, might have increased the phenomenon. Length classes from 120 to 190 mm are therefore only considered in the exponential mortality model in figure 12. Mortality is estimated for males and females separately but their values cannot be compared statistically since residual variability is not homogeneous. However, Table 5 indicates that confidence intervals are highly overlapping, suggesting that -both sexes have equivalent rates of mortality. Using this figure, we may consider that instantaneous mortality rate for all fishes is 0.0112 corresponding to a 1. 12 % loss per mm of growth. The more useful instantaneous rate of mortality in terms of ages can be estimated by multiplying the corresponding rate in terms of length by the linear rate of growth. We have Z = 0.0112 x 0.484 = 0.9954 with ages in days, corresponding to a 0.5% loss per day, 15% per month, and 86% per year. 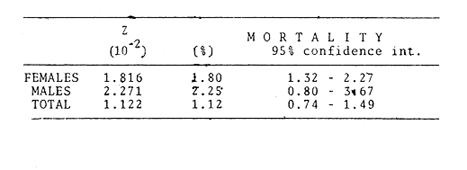 TABLE 5 INSTANTANEOUS RATE OF NATURAL MORTALITY (Z) IN RELATION TO LENGTH (MM) FOR SEABREAM MALES AND FEMALES, FROM 120 TO 180 MM STANDARD LENGTH AND CORRESPONDING 95% CONFIDENCE INTERVALS FOR MORTALITY RATES (%). Taylor (1959) stresses that natural mortality (M) must be a function of the life span and growth. The faster a fish species completes its growth pattern, the shorter is life span and thus its natural mortality must be correspondinly high. Taylos gives the following formula: M = 2.996 x K/2.996 + K to in which M = instantaneous rate of natural mortality; K and to = constants of the von Bertalanffy growth model For the seabream, we obtained M = 0. 00397 with ages in days, corresponding to a 0.3 % loss per day, 11. 4 % per month and 76.5 % per year. These results are in close agreement whith the rates calculated from the observed catch curve. Chavance et al. (1983) obtained from egg and larval survey in the same area the following natural mortality estimates for the seabream: Egg stage --- 15.5 days old larva --- 35 % per day 15.5 days --- 32 days old larvae --- 16.7% per day 32 days --- 1 year old fishes --- approximately 0. 2 % per day Their ultimate estimation is in agreement with our result, which is 0.5%. Therefore, juvenile and adult data confirm their conclusion that the major part of a year-class mortality occurs during the very first days of planktonic life. The evolution of a cohort weight may be estimated from the growth and mortality processes. Table 6 gives the corresponding data for a cohort made of 10,000 individuals when 1 month old. We see that the cohort biomass reaches its maximum value with 7 months old. After this socalled critical age, biomass is decreasing since gains due to fish growth do not counterbalance losses due to fish mortality. 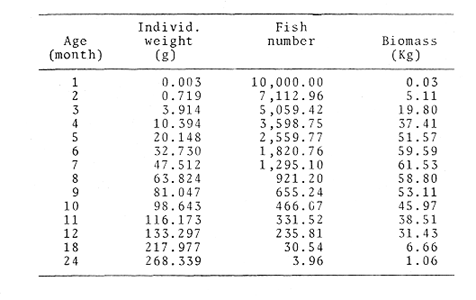 TABLE 6 COHORT BIOMASS EVOLUTION ACCORDING TO SEABREAM GROWTH AND MORTALITY Potential yield, i.e., how much can be taken each year from the stock on a sustained basis, may be estimated through the formula given by Gulland (1975) for unexploited stocks: Y = 0.5 M Bo in wich: Y = potential yield; M = natural mortality coefficient; Bo = unexploited standing stock. With regards to seabream and considering that M = 1.971 and Bo = 2,500 metric tons, we conclude that potential yield is 2,464 tons. In conclusion, it appears that this successful estuarine fish species has a great niche size. It is characterized as a trophic generalist when its whole life cycle is considered, and utilizes a wide range of habitats. Its life cycle is greatly shortened when compared to systematically related species; its biomass and production are correspondingly high. Information on ecology, biology and population dynamics of a particular species belonging to a certain community are important for a better utilization of such a biological resource by man, and are also valuable since it constitutes the biological basis of the community structure and evolution. Therefore, future research must be directed towards such information on more species and also on their integration to the general knowledge of the entire ecosystem concerned. AgradecimientosACKNOWLEDGEMENTSThe institutional and financial support for the present study was provided by the Instituto de Ciencias del Mar y Limnologia, Universidad Nacional Autónoma de Mexico (ICML-UNAM). The support was also provided by the UNAM-CONACYT Project QCMACFR-011698. The logistic support was provided by the staff of the Estación de Investigaciones Marinas "El Carmen". The authors thank to A. Ayala-Castañares and A. Laguarda-Figueras for institutional support, and to J. Daget and A. Aboussovan for constructive criticisms of the manuscript. LITERATURAAMEZCUA LINARES, F., and A. YAÑEZ-ARANCIBIA An. Centro Cienc. del Mar y Limnol. Ecologia de los sistemas fluvio-lagunares asociados a la laguna de Términos. EI habitat y estructura de las comunidades de peces. Univ. Nal. Autón. Mexico 1980 69-118 (1) 76 ARIAS, A. Inv. Pesq. Crecimiento, régimen alimentario y reproducción de la dorada (Sparus aurata L.) y del robalo (Dicentrarchus labrax L.) en los estereos de Cádiz. 1980 59-83 (1) 44 ATZ, J. W. Intersexualities in Vertebrates Including Man. Intersexuality in fishes. C. N. Armstrong and A. J. Marshall Academic Press London England p. 145-232 BEVERTON, R. J. H., and S. J. HOLT A review of the lifespan and morality rates of fish in nature, and their relation to growth an other physiological characteristies. G. E. W. Wolstenholme and M. O'Connor. Ciba Foundation Coloquia on Ageing, 5. J, and A. Churchill Ltd. London England 1959 p. 142-177 BEVERTON, R. J. H. Maturation, growth and mortality of clupeid and engraulid stocks in relation to fishing. Rapp. P. V. Reun. Cons. perm. int. Explor. Mer 1963 44-67 154 BRAVO-NÚÑEZ, E., and A. YAÑEZ-ARANCIBIA An. Centro Cienc. del Mar y Limnol. Ecologia de la boca de Puerto Real, Laguna de Térmirros. 1, Descripción del área y análisis estructural de las comunidades de peces. Univ. Nal. Autón. México 1979 125-182 (1) 6 BRIGS, J. C. Bull. Fla. State Mus. Marine Zoogeography. McGraw-Hill. Co., N. Y., USA. CALDWELL, D. K., 1957. The biology and systematics of the pinfish, Lagodon rhomboides (Linnaeus). 1974 77-173 (6) 2 CARR, W. E. and C. A. ADAMS Food habits of juvenlle marine fishes occupying seagrass beds in the estuarine zone near Crystal river, Florida. Trans. Amer. Fish. Soc. 1973 511-540 (3) 102 CHAVANCE, P., C. FLORES-COTO and A. SANCHEZITURBE Early life history and adult biomass of seabream in the Terminos Lagoon, Southern Gulf of Mexico. Trans Amer. Fish Soc. 1983 166-177 113 d'ANCONA U. Détermination et differenciation du sexe chez les poissons. Arch. Anat. micro. et morph. exp. 1950 274-294 (3) 39 d'ANCONA U. Ann. Biol. Inversions spontanées et expérimentales dans le gonads de téléostéens. 1956 89-99 (3-4) 32 DARNELL, R. M. Food habits of fishes and larger invertebrates of Lake Pontchartrain, Louisiana, and estuarine community. Publ. Inst. Mar. Sci., Univ. Texas 1958 353-416 5 DE SYLVA, D. P. Biscayne Bay. Past 1 Present 1 Future. Fishes of Biscayne bay, Florida. A. Thorhang and A. Voker Symposium University of Miami 1976 p. 181-202 April 2-3, 1976. FAGADE, S. O. Ageing of Fish. Age determination in Tilapia melanotheron (Ruppell) in the Lagos Lagoon, Nigeria, with a discussion of the environmental and physiological basis of growth markings in the tropics T. B. Bagenal Unwin Brothers Ltd. England 1974 p. 71-77 FOX, L. S., and W. R. MOCK, Jr. Seasonal occurrence of fishes in two shore habitats in Barataria bay, Louisiana. Proc. Louisiana Ac. of Sci. 1968 43-53 31 GONZALEZ-SANSON, G., L. ÁLVAREZ-LAJONCHERE and M. BÁEZ-HIDALGO Lista preliminar de peces presentes en las lagunas costeras de Tunas de Zaza, Cuba. Ciencias, Ser. 8, Invest. marinas, 32. 1978 GRAHAM, D. S., I. P. DANIELS, J. M. HILL and J. W. DAY, Jr. An. Inst. Cienc. del Mar y Limnol. A preliminary model of the circulation of Terminos Lagoon, Campeche, México. Univ. Nal. Autón. México 1981 51-62 (1) 8 GULLAND, J. A. Part 5: Objetives and basic methods. Manual of methods for fisheries resources surveys and appraisal. F.A.O. Fish. Tech. Pap. 1975 1-29 145 HANSEN, D. J. Fish. Bull. Food, growth, migration and reproduction, and abundance of pinfish, Lagodon rhomboides, and Atlantic croaker, Micropogon undulatus, near Pensacola, Florida, 1963-65. 1969 135-146 (1) 68 HELLIER, T. R., Jr. Fish production and biomass studies in relation to photosynthesis in the Laguna Madre of Texas. Publ. Inst. Mar. Sci., Univ. Texas 1962 1-22 8 HILDGE, V. On the determination of stages of gonad ripeness in female bony fishes. Meeresforsch 1977 149-155 25 HOLT, S. A. Temporal and spatial distribution of fishes in the upper Galveston bay system with particular reference to the cooling water system of Cedar Bayou generating station. M. S. Thesis, Texas A & M. Univ. 1976 HOUDE, E. D. Effects of temperature and delayed feeding on growth and survival of larvae of three species of subtropical marine fishes. Mar. Biol. 1974 271-295 26 HOUDE, E. D. Effects of stocking density and food density on survival growth and yield of laboratoryreared larvae of the seabream Archosargus rhomboidalis (L.) (Sparidae). 1. Fish. Biol. 1975 115-127 7 HOUDE, E. D. Bull. Mar. Sci. Critical food concentrations for larvae of three species of subtropical marine fishes. 1978 395-411 (3) 28 HOUDE, E. D. and T. POTTHOFF Bull. Mar. Sci. Egg and larval development of the seabream Archosargus rhomboidalis (Linnaeus): Pisces, Sparidae. 1976 506-529 (4) 26 LE CREN, E. D. The length weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). 1. Anim. Ecol. 1951 201-219 20 LINDALL, W. N., Jr., 1. R. HALL and C. H. SALOMAN Fish. Bull. Fishes, macroinvertebrates and hydrological conditions of upland canals in Trampa Bay, Florida. 1973 155-163 (1) 71 LIVINGSTON, R. J. Diurnal and seasonal fluctuations of organisms in a north Florida estuary. Est. Coasts. Mar. Sci. 1976 373-400 4 LOESCH, H., J. BisHop, A. CROWE, R. KUXLYR and P. WAGNER Technique for estimating trawl efficiency in catching brown shrimp (Penaeus aztecus), Atlantic croaker (Micropogon undulatus) and spot (Leiostomus xanthurus). Gulf Res. Rep. 1972 29-33 (2) 5 NIE, N. H., C. HADLAI HULL, J. G. JENKINS, K. STEINBRENNER and D. H. BENT Statistical Package for The Social Sciences. McGraw-Hill N. Y. USA 1975 ODUM, W. E. and E. J. HEALD Bull. Mar. Sci. Trophic analyses of an estuarine mangrove community. 1972 671-738 22 ODUM, W. E. and E. J. HEALD Estuarine Research The detritus-based food web on an esturine mangrove community. L. E. Cronin Academic Press London England 1975 p. 265-286 1 OGREN, L. H. and H. A. BRUSHER The distribution and abundance of fishes caught with a trawl in the St. Andrew bay system, Florida. North. Gulf Sci. 1977 83-105 (2) 1 PANNELLA, G. Fish otoliths: daily growth layers and periodical patterns. Science N.Y. 1971 1124-1127 173 RANDALL, J. E. Food habits of reef fishes of the West Indies. Stud. trop. Oceanogr. Miami 1967 + 847 XX 5 RESÉNDEZ MEDINA, A. Estudio de los peces de la laguna de Tamiahua, Veracruz, México. An. Inst. Biol. Univ. Nal. Autón. México, ser. Cienc. del Mar y Limnol. 1970 79-146 (1) 41 RESÉNDEZ-MEDINA, A. Rev, Soc. Mex. Hist. Nat. Estudio de los peces de la laguna de Alvarado, Veracruz, México. 1973 183-281 34 RESÉNDEZ-MEDINA, A. Lista preliminar de peces colectados en las lagunas de Nichupté y Bojórquez, Cancún. Quintana Roo, México. An. Inst. Biol. Univ. Nal. Autón. México ser. Zoologia 1975 87-100 (1) 46 RICKER, W. E. Bull. Fish. Res. Bd. Can. Computation and interpretation of biological statistics of fish population. 1975 1-382 191 SABINS, D. S. and F. M. TRUESDALE Diel and seasonal occurrence of immature fishes in a Louisiana tidal pass. Proc. 28th An. Conf. South. Ass. Came and Fish Com. 1974 161-171 SIMMONS, E. G. and H. D. HOESE Studies on the hydrography and fish migrations of Cedar Bayou, a natural tidal inlet on the central Texas coast. Publ. Inst. Mar. Sci., Univ. Texas 1959 56-80 6 SPRINGER, V. G_ and K. D. WOODBURN An ecological study of the fishes of the Tampa Bay area. Fla. State Bd. Conserv. Mar. Lab., Prof. Pap. 1960 1-104 1 STEPIEN, W. P. Feeding of laboratory reared larvae of the seabream Archosargus rhomboidalis (Sparidae) Mar. Biol. 1976 1-16 38 SURRAHMANYAM, C. B., and S. H. DRAKE Bull. Mar. Sci. Studies on the animal communities in two North Florida salt marshes. Part 1: Fish communities. 1975 445-465 (4) 25 SWINGLE, H. A. Alabama Mar. Res. Bull. Biology of Alabama estuarine areas: Cooperative Gulf of Mexico estuarine inventory. 1971 1-123 5 TAYLOR, C. C. Cod growth and temperature. Cons. 1958 366-370 (3) 23 TAYLOR, C. C. Temperature and growth - The Pacific razor clams. J. Cons. 1959 93-101 (1) 25 VAUGHAN, F. A. Food habits of the seabream, Archosargus rhomboidalis (Linnaeus), and comparative growth on plant and animal food. Mar. Sci. 1978 527-536 (3) 28 WINDELL, J. T_ and S. H. BOWEN Methods for Assessment of Fish Production in Fresh Waters. Methods for study of fish diets based on analysis of stomach contents T. Bagenal Blackwell Scientific Publications Oxford England 1978 p. 219-226 YAÑEZ-ARANCIBIA, A. and J. W. DAY, Jr. Coastal Lagoon. Ecological characterization of Terminos Lagoon esturine system in the Southern Gulf of Mexico P. Lasserre and H. Postma Oceanologica Acta. 1982 p. 431-440 (4) 5 YAÑEZ-ARANCIBIA, A., F. AMEZCUA LINARES and J. W. DAY, Jr. Estuarine Perspectives. Fish community structure and function in Terminos Lagoon, a tropical estuary in the southern Gulf of México V. Kennedy Academic Press N. Y. USA 1980 p. 465-482. YAÑEZ-ARANCIBIA, A., A. L. LARA-DOMINGUEZ, P. SANCHEZ-GIL, I. VARGAS MALDONADO, P. CHAVANCE, F. AMÉZCUA LINARES, A. AGUIRRE LEÓN and S. DIAZ RUIZ Coastal Lagoons. Ecosystem dynamics and nichthemeral and seasonal programming of fish community structure in a tropical estuarine inlet, México P. Lasserre and H. Postma Oceanologica Acta. 1982a p. 417-429 (4) 5 YAÑEZ-ARANCIBIA, A., F. AMEZCUA LINARES and M. TAPIA GARCIA An. Inst. Cienc. del Mar y Limnol. Prospección ictioecológica del estuario del Rio Champotón, Campeche, México Verano 1979. Univ. Nal. Autón. México 1982b 395-398 (1) 9 YAÑEZ-ARANCIBIA, A., A. L. LARA-DOMÍNGUEZ, P. CHAVANCE and D. FLORES HERNANDEZ An. Inst. Cienc. del Mar y Limnol. Environmental behaviour of the ecological system of Terminos Lagoon, Campeche, México. Univ. Nal. Autón. México 1983 137-176 (1) 10 ZUÑIGA, L. R. Estudio del crecimiento de Boops boops (L.) del Levante español. Inv. Pesq. 1967 383-418 (3) 31
|

