|
RESPIRATION STUDIES IN A LOUISIANA SALT MARSH
Trabajo
recibido el 28 de abril de 1977 y aceptado para su publicación el 24 de
junio de 1977.
CHARLES S. HOPKINSON
JOHN W. DAY, JR
B. T. GAEL
Dept. Marine Sciences, Center for Wetland Resources,
Louisiana State University, Baton Rouge, LA 70803 USA. This work is a result of
research sponsored by NOAA Office of Sea Grant, Department of Commerce, under
Grant N° 04-3-158-19.
Desde julio de 1973 hasta Agosto de 1974 se realizaron
estudios sobre respiración en un pantano salobre de Louisiana. El
oxígeno disuelto de la zona intertidal del pantano (aquella zona mojada
por las mareas) fue medido en una caja colocada sobre el pantano. La
respiración de la superficie del pantano fue medida, mediante el
oxígeno disuelto de la capa de agua que cubre el lodo dentro del
núcleo de muestra y en los sedimentos sumergidos fue determinado
in situ utilizando un respirómetro "bell-jar". La
oxidación química y la respiración bacterial en el
núcleo de muestra fue estimada usando formalina y antibióticos,
respectivamente. La respiración total promedio de la zona intertidal,
del lodo superficial del pantano y de los sedimentos sumergidos fue de 2.3,
0.44 y 1.3 g 02/m²/día, respectivamente. La respiración
bacterial alcanzó el 51% de la muestra biológica total de la
superficie del pantano. Cerca del 80% de la respiración intertidal total
se debió a la comunidad epibiótica de los tallos de los pastos.
La profundidad del agua en el pantano pareció ser el principal factor
que controla la respiración intertidal. Los resultados sobre la
respiración de los sedimentos sumergidos sostienen la tesis de que la
respiración béntica es una función del aporte de la
materia orgánica. El presupuesto de carbón, calculado del pantano
salobre, indica un exceso de producción que no puede medirse ya sea por
consumo o por exportación. Creemos que esta exportación se
deposita en los sedimentos.
Respiration studies of the salt marsh intertidal zone
and adjacent submerged sediments were carried out in coastal Louisiana from
July 1973 to August 1974. Respiration of the marsh intertidal zone was measured
by dissolved oxygen uptake in an enclosure over the marsh. Marsh sediment
surface respiration was measured in water overlying sediment cores. Chemical
oxidation and bacterial respiration in the marsh sediment cores were
partitioned from total uptake using formalin and antibiotics, respectively.
Submerged sediment oxygen uptake was determined in situ
using bell-jar respirometers. Marsh intertidal zone respiration
averaged 2.3 g 0, m-² day-¹. Surficial marsh sed¡ments and
submerged lake sediments averaged 0.44 and 1.3 g 02 m-² day-¹,
respectively. Bacterial respiration accounted for 51% of total biological
uptake on the marsh sediment surface. Roughly 80%, of the total respiration in
the marsh intertidal zone was due to the epibiota on marsh grass stalks.
Regression analyses indicated water depth on the marsh was the dominant factor
controlling respiration in the intertidal zone. Spatial variations in submerged
sediment respiration suggested that benthic respiration is a function of
organic matter supply. A carbon budget of the salt marsh ecosystem indicated
excess production not accountable for by consumption or export may he deposited
in submerged sediments.
Along Louisiana's Gulf Coast lies one of the largest and most productive marsh systems in the world. The rate of net production in these marshes is as high as that of intensively cultivated crops such as sugarcane and rice. Extremely high net production of the saline marsh grass Spartina alternif lora has been measured in Georgia (Schelske and Odum, 1962; Teal, 1962) and Louisiana (Kirby and Gosselink, 1976). The detrital-based food web. The importance of organic detritus in estuarine environments has been discussed by Darnell (1958, 1961), Teal (1962), ScheIshe and Odum (1962), Odum and de la Cruz (1967), Heald (1971), W. E. Odum (1971), and Day et al., (1973). Salt marsh ecosystems can be divided into two functional strata: an upper canopy of leaves where photosynthesis exceeds respiration, and a lower zone of net consumption. Consumers in the upper zone are a small, transient group. The lower zone in Louisiana salt marshes is typically between the tides (intertidal) and is dominated by detritus decomposers and consumers, such as protozoa, bacteria and fungi, meiofauna, and macrofauna. Most outer culms of marsh grass stalks in this intertidal zone are dead so photosynthesis is by epipelic mud algae and epiphytic algae. This epiphytic community is heterotrophic (Stowe, 1972). Respiration of the intertidal zone has been measured in Georgia (Teal, 1962; Teal and Kanwisher, 1961) and New England (Nixon and Oviatt, 1973) but no measurements have been reported for the Gulf of Mexico. The purpose of this study was to measure the respiration of this intertidal community and the adjacent submerged sediments. A more general objective was to determine the role of the salt marsh in overall estuarine carbon flow. DESCRIPTION OF THE AREARespiration studies were conducted in a salt marsh estuary along the western margin of Caminada Bay (Fig. l). Measurements were made of submerged sediments of Airplane Lake, a shallow 17.6 ha saline lake, and of the surrounding salt marsh intertidal zone. Wave and tidal energy in this basin is low with a mean daily tidal range of about 0.3 m. Seasonal variation in mean sea level is about 0.3 m with lowest mean water levels occurring in winter. This seasonal variation causes the marsh to flood deeper, more often and for longer periods during the warmer months of the year. A natural levee surrounds the lake with a crest height of about 18 cm above mean sea level (MSL) occurring about 4-5 meters from the shore. The dominant marsh macrophyte is Spartina alterniflora. More detailed description of the area are given elsewhere (Frazier, 1967; Gagliano and van Beek, 1970; Day et al., 1973; and Kirby and Gosselink 1976). Respiration studies of the submerged sediments were carried out within 5 m of the shore and in the middle, about 60 meters from shore, of Airplane Lake. Respiration of the salt marsh intertidal zone, which includes the soil surface and in the middle, about 60 meters from shore, by tides, was measured within 10 m of the lake edge. MATERIALS AND METHODSRespiration of the salt marsh intertidal community and adjacent submerged sediments was determined by measuring oxygen uptake utilizing three methods: 1) a core method for the marsh floor community, 2) a "box" method for the respiration of the entire intertidal marsh community, and 3) a "bell-jar" technique for submerged lake sediments. Dissolved oxygen was measured using a portable battery-powered Martek Model DOA Dissolved Oxygen Monitoring System, with a temperature compensated polarographic oxygen electrode with a teflon membrane. Measurements were taken monthly from July 1973 until August 1974.  Fig. 1. Map. of study area showing the location of Airplane Lake where respiration studies were carried out. CORE METHODCore respiration was measured at night in a small floating laboratory anchored adjacent to the marsh. Within an hour before darkness twelve marsh mud cores were taken from bare mud arcas of the natural streamside levee surrounding Airplane Lake using aluminum tubing (7.3 cm I. D.). Care was taken to avoid arcas where large dead Spartina or Distichlis stems and leaves, crab burrows, or larger macrofauna were present as the pressure of the tube on pieces of grass disturbed the sediment surface exposing deeper anoxic material. The community sampled consisted of algae, detritus and bacteria, microfauna, and meiofauna living in and on the mud. Marsh sediment cores were then transferred in a styrofoam container to the floating lab. Fourhundred ml of water taken from Airplane Lake were added to each core so that the mud was covered with about 9 cm of water. The water was added such that there was minimum disturbance of the surface mud. The styrofoam container was filled with Airplane Lake water to keep the cores at ambient temperature. At the same time, four opaque BOD bottles were filled and incubated in the lake. Because of some initial, rapid, chemical uptake of oxygen by reduced materials exposed during the coring process, 30 minutes was allowed to elapse before beginning dissolved oxygen measurements (Pamatmat and Banse, 1969; Teal and Kanwisher, 1961). To partition chemical oxidation from total oxygen uptake, sufficient formaldehyde was added to 4 of the cores to make a 5% final concentration (Teal and Kanwisher, 1961; Hargrave, 1969a, 1969b, 1970; Pamatmat, 1968, 1971; Smith, 1973, 1974). Bacterial respiration was estimated by difference in oxygen uptake in antibiotic-treated cores and untreated cores, using a method similar to that of Hargrave (1969a). Sufficient amounts of antibiotics were added to four cores to give 50 mg 1-¹ final concentration of both penicillin G and streptomycin sulfate. This method may underestimate bacterial respiration, because this concentration bacterial activity may not be completely inhibited. However it is proven not to affect activity of most eukaryotes (Oppenheimer, 1955; Marshall and Orr, 1955; Droop, 1967). The remaining four cores with no addítives served as controls. After the addition of formaldehyde and antibiotics, dissolved oxygen was measured at 0.5 hours and then hourly for four hours. The water in the cores was gently stirred each half hour so that no oxygen gradient developed in the water column but not vigorously enough to disturb the sediment surface (Teal and Kanwisher, 1961; Hargrave, 1969a; Edwards and Rolley, 1965). BOX METHODRespiration of the intertidal communiy was measured by in situ oxygen uptake in a box covering 0.25 m² of marsh including all parts of the intertidal community the boxes were 0.8 m high and 0.5 m on a side and had sheet metal around the bottom edge which enabled them to be pushed into the mud. Teal and Kanwisher (1961) used similar enclorures in a Georgia salt marsh for studies of gas exchange when the marsh for studies of gas exchange when the marsh was not flooded. During each measurement, two boxes were placed in the marsh within 10 m of the waters edge. Four to six replicates were taken each month. The boxes were normally set out at slack high tide so that measurements took place on the ebbing tide. Because of the loosely attached sheathing, the water level in the box remained the same as outside. Measurements were made when ther was a minimum of 6 cm of water over the marsh. When tide was high during the day, the obxes were fitted with light-tight covers. To minimize overheating during these measurements, the boxes were painted high gloss white and fitted with ventilation fans. This prevented the water tempeature in the box from wxceeding that outside. Dissolved oxygen and temperature were recorded continuosly for 4 to 6 hours. Two BOD bottles were carefully filled with water from within the box for the duration of the measurement. Water depth in and out of the boxes was measured periodically throughout the experiment. MEASUREMENTS OF BENTHIC COMMUNITY RESPIRATIONUptake of oxygen by the benthic community was measured near the shore and in the center of Airplane Lake using in situ respirometers. The apparatus consisted of an aluminum frame to which two opaque Plexiglass cylinders (15.2 cm. I. D.) were attached. One cylinder was sealed at the bottom and the other remained open. The top of each was covered with a rubber septum with openings for oxygen electrodes and wire-loop stirrers. The entire apparatus was suspended on a 2.5 m pole. A collar allowed the open ended cylinder to penetrate 10 cm into the mud. The suspending pole extended about 70 cm into the mud to steady the apparatus. Experiments were conducted at night using duplicate respirometers at each lake site. Care was taken when placing the respirometers to assure that the sediment was disturbed as little as possible and that the open-ended cylinder was vertically aligned. Oxygen uptake was measured for 4 to 6 hours. The water in each cy1inder was gently stirred every 30 minutes and oxygen was measured every 60 minutes. An initial 30 - 60 minute waiting period was allowed for any chemical oxidation of disturbed sediments to take place. On one occasion during the study chemical uptake of oxygen by the sediments was measured by comparing oxygen uptake in the two respirometers after poisoning one with sufficient formaldehyde to reach a 5% final concentration. In comparison to the cores chemical oxidation was less of a problem because of the larger cross sectional area of the respirometers and the measurements were in situ. CALCULATION OF OXYGEN CONSUMPTION RATESFor the cores dissolved oxygen measurements were averaged for the four replicates of each treatment. Rate of oxygen uptake of the BOD boxes was subtracted from the average rate of change for each treatment leaving the uptake rate by the mud surface for each tratment, where: C = rate of oxygen uptake in control cores. F = rate of oxygen uptaken in formaldehyde treated cores. A = rate of oxygen uptake in antibiotic treated cores. Total biological respiration (TBR), bacterial respiration (BR), and nonbacterial biological respiration (NBR) were then calculated as follows: (1) TBR = C - F (2) BR = TBR - (A - F) (3) NBR = TBR - BR For each bot total oxygen up, take was corrected for oxygen uptake in BOD bottles. The total amount of oxygen available for respiration was a function of volume of water in the boxes and the continnously changing water volumen was considered. Average volume gave the same result as integrating the uptake rate over small volume increments. Benthic oxygen consumption was obtained by subtracting the uptake rate in closed cy1inder from that of the exposed cylinder. RESULTS
OXYGEN CONSUMPTION OF THE MARSH SEDIMENT SURFACEThere was no clearly discernible seasonal pattern in total biological respiration of the marsh sediment surface (Table 1, Fig. 2). Total biological oxygen uptake by marsh sediments ranged from 0.85 g m-² day -¹ in September to 0.19 g m-² day-¹ in February. Uptake rates generally seemed to be lowest during the winter and early summer months. Total annual biological respiration of the sediment surface was 164 g O2 m-² (0.44 g m-² day-¹). Total oxygen uptake (chemical plus biological) ranged from 1.59 g m-² day-¹ in September to 0.19 g m-² day-¹ in February (Table l). Chemical oxidation comprised 0 - 74% of total uptake. Generally, chemical oxidation seemed to be less when the marsh surface was drier at the time the sample was taken. Calculated bacterial respiration ranged from 0-05 g O2 m-² day-¹ in December to 0.51 g O2 m-² day-¹ in August, accouhting for 11 - 100% of the total biological respiration. Calculated nonbacterial respiration ranged from zero in November to 0.48 g O2 m-² day-¹ in September averaging 49% of total biological respiration. Annually, bacterial and nonbacterial respiration rates were calculated to be 86.5 and 82.5 g 02 m-² yr-¹, respectively. Because of the high amount of variability, the differences between bacterial and nonbacterial respiration were not statistically significant. Water temperature in the cores ranged from 11° C to 32 ° C. Simple correlation tests showed no significant relation (p< .05) between water temperature and total biological respiration (r = 0.46) , bacterial respiration (r = 0.30), or nonbacterial respiration (r = 0.24) . Initial dissolved oxygen concentrations were normally slightly below saturation levels. Final oxygen concentrations were above 5 mg 1 -¹ except for May through August. Only during August did the final oxygen level fall below 3 mg 1-¹. OXYGEN CONSUMPTION OF THE MARSH INTERTIDAL COMMUNITYIntertidal respiration was greatest during spring and summer (3.90 g O2 m-² day-¹ in July, 5.06 g O2 m-² day-¹ in May) decreased through fall, and reached a low in December at 0.93 g O2 m-² day-¹ (Fig. 2). Monthly variability ranged from 1 to 85%, of the mean suggesting a very spatially heterogeneous community. 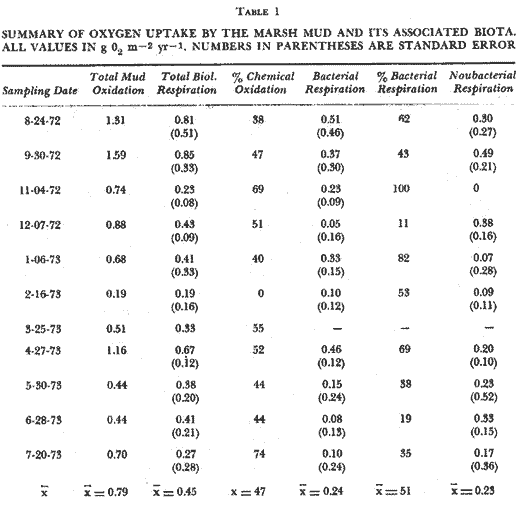 TABLA 1 SUMARY OF OXIGEN UPTAKE BY THE MARSH MUD AND ITS ASSOCIATED BIOTA. ALLVALUES IN g02 m-2 yr -1, NUMBERS IN PARENTHESES ARE STANDARERROR Considerable change in the physical appearance of the marsh during the course of this study, correlated with equally large changes in respiration, especially between February 15 and March 24. In February about 95% of the standing Spartina was dead, and thick carpets of Enteromorpha and Ectocarpus were present. In March, temperature and mean sea level were higher. Young Spartina shoots were sprouting, making the marsh approximately 50%, dead standing and 50% live Spartina shoots. Winter algal forms were almost completely gone. Respiration rates over this period increased from 1.36 g O2 m-² day-¹ in February to 2.53 g O2 m-² day-¹ on March 10, and reached 3.19 g O2 m-² day-¹ by March 24. Initial dissolved oxygen concentrations in the water over the marsh were higher during the winter months than during the summer, fall and spring months. During the warmer months initial dissolved oxygen concentrations were o ften below 5 mg 1-¹ and often went below 1 mg 1¹-. When oxygen reached one mg 1-¹, experiments were terminated. Respiration rate was regressed linearly against water temperature, water depth, and temperature and depth together. The results indicated a rather minor influence of temperature on respiration rates (R² = .35, p < .05) . There appeared to be an inhibitory effect of temperature on respiration at temperatures higher than 23°C but the relationship was not statistically significant. Water depth over the marsh and monthly changes of depth were significantly and positively correlated with respiration rates especially during the winter when low mean sea levels resulted in extended periods without tidal inundation of the marsh. During November to March water depth and monthly water depth changes accounted for 83% (R ² = .83, p < .05) and 85% (R² = .85, p< .01) of the observed variation of respiration rates and monthly respiration changes, respectively. Yearly, however, monthly water level changes accounted, for only 46% (R² = .46, p< .05) of the variation in monthly respiration changes. 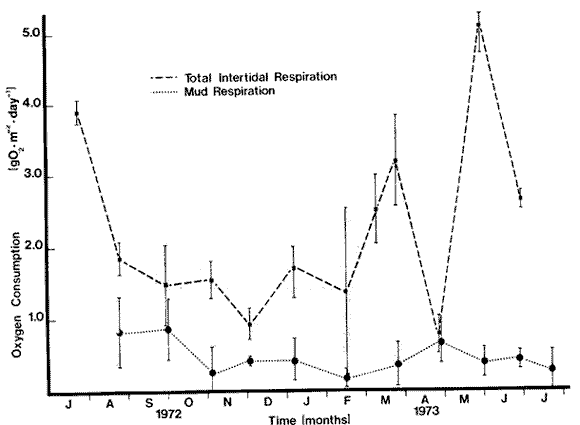 Fig. 2. Comparison of oxygen consumption by the entire intertidal marsh community and the mud surface alone. Vertical bars are standard error. RESPIRATION OF THE SUBMERGED BENTHIC COMMUNITYTotal oxygen uptake (biological and chemical) by sediments ranged from 0.61 to 2.09 g O2 m-² day-¹ in the mid-lake area and from 1.03 - 2.74 g O2 m-² day-¹ along the edge (Fig. 3). Variation between duplicate measurements was large in both areas as shown by the magnitude of the standard error of the mean suggesting a rather heterogeneous bottom. Monthly variation within areas was often as great as between areas. During winter (NovemberMarch) and on a yearly basis there were no significant differences between areas. Respiration was 36% lower in the middle of the lake during the winter than along the lake edge. Rates were lower during winter (Nov-Mar) than during the rest of the year in the mid-lake area (1.58 ± 0.15, vs 0.94 ± 0.13 g O2 m-² day-¹ but not in the edge area (1.67 ± 0.22 vs 1.48 ± 0.28 g O2 m-² day-¹. These data area mean ± S.E.) . Annually, respiration averaged 472 ± 51 g O2 m-² midlake and 580 ± 62 g O2 m-² lake edge. Water temperature changed seasonally ranging from 32° C to 13° C in the center and from 32° C to 11° C along the edge. Initial dissolved oxygen concentrations were generally above 6 mg O2 1-¹ and the final concentration only went below 4 mg 1-¹ once. 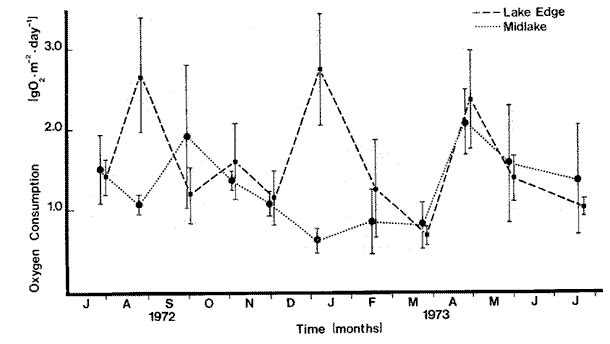 Fig. 3. Oxygen consuption of submerged sediments in Airplane Lake. Vertical bars are Standard error. The design, of the respirometers made the use of formalin difficult and somewhat questionable and for that reason it was added but once. Seventeen percent of the total sediment oxygen uptake was chemical in March. Assuming this percentage holds for the entire vear, total biological uptake of oxygen on the lake bottom was 1.07 and 1.32 g O2 m-² day-¹ in the center and along the edge respectively. As on the marsh, temperature accounted for little of the observed yearly variation in respiration. Linear regression analyses of temperature on respiration showed higher correlation coefficients at temperatures below 22°C in both the mid-lake and lake edge areas (R² = .24, p< .05 and R² = 13, p<. 1). This suggested that higher temperature may have an inhibitory effect on respiration. DISCUSSION
SALT MARSH RESPIRATIONTotal interdidal respiration was 823 g O2 m-² yr-¹ or an average of 2.3 g O2 m-² day-¹. An intertidal respiration rate of. 3.9 g O2 m-² day-¹ measured by Teal and Kanwisher (1961) during spring in Georgia compares quite favorably with rates noted in this study during the same time of year. Water depth over the marsh seems to be the overriding factor controlling respiration in the intertidal zone. Respiration rates were significantly and positively correlated with water depth and water depth changes but not to temperature. This strong correlation with water level reflects the fact that most biomass is located on the stalks and as water level increases more epíphytic community is inundated. The greatest populations of bacteria and epiphytic algae are found on the wetted portions of the marsh grass (Hood, 1970; Stowe, 1972). Epibiota on the stalks was responsible for about 80% of the total intertidal biological oxygen uptake. Teal and Kanwisher (1961) found that this epiphytic community in a Georgia salt marsh accounted for 55%, of the total intertidal oxygen uptake (including chemical oxygen demand). If chemical oxygen demand of the mud surface in Georgia is similar to this study's, then the epiphytic community on Spartina stalks in Georgia would amount to about 70% of the total intertidal respiration. The average annual biological oxygen uptake by the intact mud surface in this study was 0.45 g O2 m-² day. Comparable annual rates for Georgia are 0.8 (Pomeroy, 1959) and 2.3 (Duff and Teal, 1965), and 3.0 for Nova Scotia (Duff and Teal, 1965). A rate of 1.7 was measured in late spring-early summer in Georgia (Teal and Kanwisher, 1961). SUBMERGED BENTHIC RESPIRATIONAverage oxygen uptake of submerged sediments in Airplane Lake (1.3 g O2 m-² day-¹) is in the upper range of reported values (0.02 - 2.8g O2 m-² day-¹) (Table 2). Edwards and Rolley (1965) and Smith (1973) reported oxygen uptake values nearly twice those of this study. Short time periods of Smith's measurements (one hour) could have accounted for his high readings as higher initial rates were observed in these experiments. 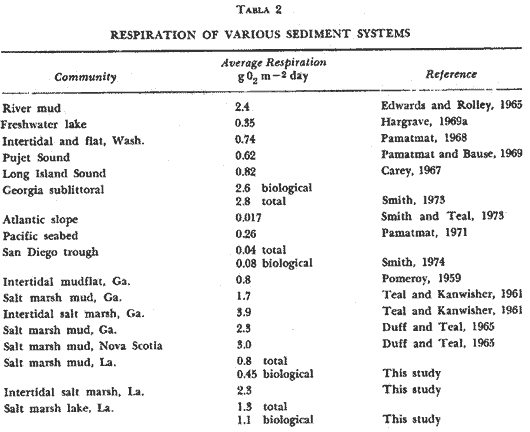 TABLA 2 RESPIRATION OF VARIOUS SEDIMENT SYSTEMS Respiration and temperature were poorly correlated in this study. Several researchers (Pamatmat and Banse, 1969; Duff and Teal, 1965; Hargrave, 1969b; Smith, 1973, 1974) have suggested the magnitude and temporal patterns of benthic respiration are a function of the rate of supply of organic material. Smith attributed the high rates of benthic respiration to the high productivity and export of organics in the Georgia salt marshes. Respiration rates shown in Table 2 support an oxygen uptake-organic matter relationship. Generally, the closer a site is to a large organic input, the higher the respiration rate, whether it is sewage as in the bay study by Smith et al. (1973), or as salt marsh detritus as in our study. On a smaller scale Pamatmat and Banse (1969) were led to the same conclusion in a study in Puget Sound. They felt that seasonal patterns of oxygen uptake were due to changing supplies and inputs of organic matter. In freshwater studies, Edwards and Rolley (1965) and Hargrave (1969a, b) suggested a correlation between respiration and dissolved oxygen concentration that is lacking in marine studies (this study, Pamatmat, 1968; Pamatmat and Banse, 1969; Smith, 1973). Final oxygen levels reached in our core experiments seemed to, have little effect on respiration. In August, 1972, the dissolved oxygen concentration fell steadily to 0.8 mg 1-¹ by the end of the experiment, and this was the second highest rate measured. When using the boxes to measure respiration we found that not until the dissolved oxygen level fell below 1 mg 1 - ¹ did respiration decrease noticeably. The facultative nature of many of the consumers may account for this phenomenom. BACTERIAL AND CHEMICAL OXYGEN CONSUMPTIONBacterial respiration, estimated from antibiotic studies, accounted for 51 % of total biological oxygen uptake of the marsh surface. Hargrave (1969a) using the same techniques, estimated that bacterial respiration of benthic sediments of Marion Lake, British Columbia, was between 39-45% of total respiration. Kanwisher (1962) estimated that bacterial respiration was 70% of the oxygen consumption in marine sediment cores. Smith et al. (1973) determined the percentage of community respiration attributable to bacteria in a study of benthic community respiration near and distant from a sewage outfall near, Woods Hole. Near the sewage outfall bacterial respiration was 64% of the total biological oxygen consumption, while at the Buzzards Bay control site bacteria accounted for 43% of community respiration. In the sublittoral zone off the Georgia coast Smith (1973) estimated bacteria consumed 30 - 60% of the biological uptake of oxygen. Smith's (1974) study in the San Diego trough showed bacteria comprised 16% of total biolog¡cal uptake. Smith (1973 and 1974) and Smith et al. (1973) suggested that high magnitudes and proportions of bacterial and chemical oxygen demand or high biological rates of respiration were correlated with high inputs of organic matter. The results from this study and others support the bacterial-organic input relationship. At one extreme is the San Diego trough which is an area of small input where bacteria account for only 16% of total respiration. At the other extreme are rich coastal sublittoral and marsh systems which have high proportions of biological respiration attributable to bacteria. There are conflicting results concerning the relationship between chemical oxygen demand and organic input. The Georgia sublittoral, for example, is an area of high organic input rates, but only 5 - 9% of total oxidation is attributable to chemical demand. In contrast the chemical oxidation in the San Diego trough made up as large a percentage of total oxygen uptake as reported here. There are questions concerning the use of antibiotics to measure bacterial respiration. Due to the charged nature of clay and detrital particles in the cores, adsorption of the antibiotics may lead to higher concentrations on the particles. Although the bacteriostatic properties of the antibiotics would be increased, the increased concentrations may affect meiofauna living and feeding on the particles. The bacteriostalis of antibiotics in brackish water may be altered due to differing abundances of certain elements such as iron. Additionally, if feeding of meiofauna involves a chemotaxis with living bacteria, then the feeding rate of the meiofauna might be changed by antibiotics. Thus the results probably do not give an absolute measure of bacterial respiration but they do enable comparisons to be made with other investigations using this method. CARBON BUDGET OF THE SALT MARSH ECOSYTEMTotal oxygen consumption by the streamside intertidal community was 815 g O2 m-² yr-¹. Results of Odum and de la Cruz (1967) indicate that combustion of 1 g of detrital material consumes 1.25 g O2. Therefore, 815 g O2 m-² yr-¹ is utilized in the combustion of 718 g organic matter m-² yr-¹. The net organic production of the streamside marsh is about 2500 g m-² yr-¹ (Kirby and Gosselink, 1976). Thus there is an excess of 1782 g organic matter m-² streamside marsh yr-¹ or about 70% of net production (see Fig. 4). By contrast, Teal (1962) in Georgia and Day et al. (1973) in Louisiana estimated that about 50% of net productivity was not consumed on the marsh. However, both of these studies concerned the whole marsh rather than just the streamside portion. It might: be expected that export would be higher for streamside marsh because tidal flushing energy is higher. Kirby (1972) found that litter bag loss rates were about 50% higher in streamside marsh than in interior marsh. Kirby and Gosselink (1976) measured net organic matter production of 1000 g m-² yr-¹ in the inland marsh. Respiration in the inland marsh was estimated to be 594 g m-² yr-¹, leaving excess of 406 g m-² yr-¹ (Day et al., 1973). After taking relative areas of streamside and inland marsh into consideration (see footnotes to Fig. 4), there is an excess of 818 g organic matter m-² average total marsh yr-¹. An organic carbon budget for water (Fig. 4) was calculated in order to deduce the fate of this excess. Gross aquatic primary productivity was estimated to be 1246 g of matter m-² yr-¹. Total demand for organic matter (respiration plus export to the Gulf of Mexico) was 1546 g m-² yr-¹ leaving 336 g m-² yr-¹ which must be supplied by import from the salt marsh. Because there is more water than marsh in the study area, organic carbon from the marsh is diluted in the water column. Thus an import of 336 g to each m² of water equals an export of 408 g from each m² of water equals an export of 408 g from each m² of marsh. There is an estimated annual excess organic production of 4 10 g per m² of marsh, which cannot be accounted for by these estimates of respiration or export to the Gulf of Mexico. The excess is most likely being sedimented in adjacent water bottoms. Price (1947) found that depths of tidal basins in Louisiana and Texas have remained relatively constant for the last few centuries in spite of varying regimes of land subsidence and sediment flow throughout the region. His data suggests tidal basins have an equilibrium depth with respect to their mean width. In subsiding basins such as the Airplane Lake arca, excess marsh production might be incorporated into lake sediments to help maintain equilibrium depth. Based on a subsidence rate of 0.9 cm per year (Day, et al. 1973), sed¡ment bulk density of 0.28 g/cc (DeLaune, et al. 1976), an organic carbon content of 6.3% in the sediments (Ho, 1971) and a correction factor relating marsh arca and water arca in this basin (See footnotes to Figure 4), the organic matter needed to maintain depth in equilibrium with sea level could be derived entirely from the excess production on the marsh. 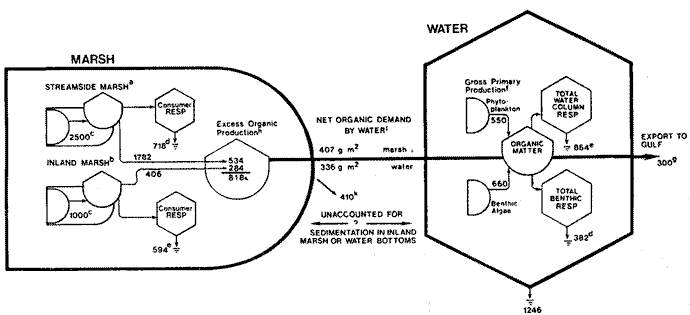 Carbon budget of the Louisiana Salt Marsh. a. Units-g organic Matter m-² streamside marsh year-¹. b. Units. Are g m-² inland marsh year-¹. c. Net above ground production of Spartina alterniflora from Kirby and Gosselink, 1976. d. This study. c. Day et al. 1973. f. Aquatic gross primary productivity estimated from Day et al. 1973 and Allen, 1975. g. Happ et al. 1977. h. Units for excess are for an average m² of marsh composed of 30% streamside marsh and 70%, inland marsh. i. All units for water are g organic matter m-² water year ¹. j. Net demand by water is total respiration plus export to Gulf minus aquatic gross primary productivity. Data for net demand by water based on 56% water and 44%, marsh. k. Units are g average m-² marsh yr-¹. AgradecimientosWe acknowIedge the assistance of Richard Day, Tom Butler, Mark Meo, Alice Simmons, james Blackmon, and Edwin Bishop. This study was supported by the Louisiana Sea Grant Program, a part of the National Sea Grant Program maíntaíned by the National Oceanic and Atmospheric Administration of the U. S. Dept. of Commerce. LITERATURACAREY, A. G., JR., . Bull. Bingham Oceanog. Coll. Energetics of the benthos of Long Island Sound. 1967 136-144 19 CAREY, F. G. and J. M. TEAL, J. Applied Physiology, Responses of oxygen electrodes to variables in construction, assembly, and use. 1965 1074-1077 20 5 DARNELL, R. M., Publ. Inst. Mar. Sci., Univ. Texas Food habits of fishes and larger invertebrates of Lake Pontchartrain, Louisiana, an estuarine community. 1958 353-416 5 Ecology Trophic spectrum of an estuarine community, based on studies of Lake Pontchartrain, Louisiana. 1961 553-568 42 DAY, .J. W., W. G. SMITH, P. WAGNER and W. STOWE Center for Wetland Resources, Louisiana State Universitv. Publ. N° LSUSG-72-04 Community structure and carbon budget in a salt marsh and shallow bay estuarine system in Louisiana. 1973 79 DELAUNE, R., W. PATRicy. and J. BRANNON Center for Wetland Resources, Louisiana State University. Publ. N° LSU-T-76-009. . Nutrient transformations in Louisiana salt marsh soils. 1976 38 DROOP, M. R., Br. Phycol. Bull., A procedure for the routine purification of algal cultures with antibiotics. 1967 295-297 3 2 DUFF, S. and J. M. TEAL, Limnol. Oceanogr., Temperature change and gas exchange in Nova Scotia and Georgia salt marsh muds. 1965 67-73 10 EDWARDS, R. W. and H. ROLLEY, J. of Ecology, Oxygen consumption of river muds. 1965 1-19 53 1 FRAZIER, D. E., Trans. Gulf coast Assoc. Geol. Soc., Recent deltaic deposits of the Mississippi River; their development and chronology. 1967 287-315 17 GAGLIANO, S. M. and J. L. VAN BEEK Coastal Resources Unit, Louisiana State University, Report N° 1 Hydrologic and geornorphic aspects of deltaic processes, Mississippi delta system. 1970 140 HAPP, G., J. G. GOSSELINK and J. W. DAY, JR., Estuar. Coast. Mar. Sci. The seasonal distribution of organic carbon in a Louisiana estuary. 1977 695-705 5 HARGRAVE, B. T., J. Fish. Res. Bd. Epibenthic algal production and community respiration in the sediments of Marion Lake. 1969 2003-2026 26 Limnol. Oceanogr., Similarity of oxygen uptake by benthic communities 1969b 801-805 14 Limnol. Oceanogr., The effect of a deposit-feeding amphipod on the metabolism of benthic microflora. 1970 21-30 15 1 HEALD, E. J., Sea Grant Tech. Bull. University of Miami . The production of organic detritus in a South Florida estuary. 1971 110 6 HO, C., Center for Wet1and Resources, Louisiana State University, Baton Rouge, Louisiana. Coastal Studies Bull. Seasonal changes in sediment and water chemistry in Barataria Bay. 1971 67-84 6 HOOD, M. A., A bacterial study of an estuarine environment: Barataria Bay. M. S. Thesis. Louisiana State University. 1970 94 KANWISHER, J. Occ. Publ. Grad. Sch. Oceanogr., Univ. Rhode Island., Gas exchange of shallow marine sediments. In: Symposium on the environmental chernistry of marine sediments. 1962 13-19 1 KIRBY, C. J., Ph. D. dissertation, Louisiana State University. The annual net primary production and decomposition of the salt marsh grass Spartina alterniflora Loisel in the Barataria Bay estuary of Louisiana. 1972 81 KIRBY, C. J. and J. G. GOSSELINK, Ecology Primary production in a Louisiana Gulf and Spartina alterniflora marsh. 1976 1052-1059 57 5 MARSHALL, S. M. and A. P. ORR, J. Mar. Res., Some uses of antibiotics in physiological experiments in sea water. 1955 341-346 17 NIXON, S. W. and C. A. OVIATT, . Ecol. Monogr., Ecology of a New England salt marsh. 1973 463-498 43 4 ODUM, E. P. and A. A. DE LA CRUZ, Ass. Adv. Sci. Publ., Particulate organic detritus in a Georgia salt marsh-estuarine ecosystem. In: Estuaries, G. H. Lauff 1967 383-388 83 ODUM, W. E., Sea Grant Tech. Bull., University of Miami, Pathways of energy flow in a South Florida estuary. 1971 162 7 OPPENHEIMER, C. H., Copeia The effect of marine bacteria on the development and hatching of pelagic fish eggs, and the control of such bacteria by antibiotics. 1955 43-49 1955 PAMATMAT, M. M., Int. Rev. Gesamten Hydrobiol., Ecological metabolism of a benthic community on an intertidal sandflat. 1968 211-298 53 Limnol. Oceanogr., Oxygen consumption by the seabed. IV. Shipboard and laboratory experiments. 1971 536-550 16 3 PAMATMAT, M. M. and K. BANSE, Limnol. Oceanogr. Oxygen consumption of the seabed. II. In situ ineasurements to a depth of 180 m. 1969 250-259 14 POMEROY, L. R., Limnol. Oceanogr., Algal productivity in the salt marshes of Georgia. 1959 367-386 4 PRICE, W. A., Bull. Amer. Ass. Petrol. Geol., Equilibrium of form and forces in tidal basins of coasts of Texas and Louisiana. 1947 1619-1663 31 SCHELSKE, C. L. and E. P. ODUM Proc. Gulf Caribb. Fish Inst., Mechanisms maintaining high productivity in Georgia estuaries. 1962 75-80 14 SMITH, K. L., Ecology, Respiration of a sublittoral community. 1973 1065-1075 54 5 SMITH, K. L., Limnol. Oceanogr., Oxygen demands of San Diego Trough sediments: an in situ study. 1974 939-944 19 4 SMITH, K. L. and J. M. TEAL, Science, Deep-sea benthic community respiration: An in situ study at 1850 meters. 1973 282-283 179 SMITH, K. L., G. T. ROWE and J. A. NICHOLS, Estuarine and Coastal Marine Science, Benthic community respiration near the Woods Hole sewage outfall. 1973 65-70 1 STOWE, W. C., Community structure and production of the epiphytic algae in the Barataria Bay area of Louisiana. Ph. D. dissertation, Louisiana State Universitv. 1972 TEAL, J. M., Ecology Energy flow in the salt marsh ecosystem of Georgia. 1962 614-624 43 4 TEAL, J. M. and J. KANWISHER, Limnol. Oceanogr., Gas exchange in a Georgia salt marsh. 1961 388-399 6 4
|

