|
AN ECOLOGICAL STUDY OF MODIOLUS DEMISSUS GRANOSSISSIMUS
SOWERBY IN LOUISIANA (USA)
Trabajo recibido el 28 de abril de 1977 y
aceptado para su publicación el 13 de junio de 1977.
DANIEL RODRÍGUEZ-ORTEGA
Instituto Nacional de Pesca. Guayaquil,
Ecuador.
JOHN W. DAY, JR.
Dept. Marine Sciences, Center for Wetland Resources,
Louisiana State University, Baton Rouge, LA 70803 USA. This work is a result of
research sponsored by NOAA Office of Sea Grant, Department of Commerce, under
Grant No 04-3-158-19.
Desde septiembre de 1973 hasta mayo de 1974 se
realizó un estudio ecológico de Modiolus
demissus. La biomasa promedio en el área estudiada fue de 6.5
g peso seco/m². La biomasa más alta fue 49.9 g/m² y la
más baja 0.5 g/m². La biomasa total se relacionó más
al tamaño del molusco que al número total. Estos moluscos forman
pequeñas colonias de 0.2 a 0.3 m de diámetro. La densidad
promedio fue 27.8 individuos/m². No hay preferencia en la
orientación del sifón. M. demissus se
encontró desde la costa del Golfo hasta aguas interiores
extendiéndose en los pantanos salobres. Los valores de salinidad anual
de estas áreas variaron desde 29.4 a 4.3 %o. No fueron encontrados estos
moluscos en pantanos intermedios; en pantanos salinos se encontraron
principalmente asociados con Distichlis spicata y Spartina
patens. En pantanos salobres se encontraron principalmente asociados
con Juncus roemerianus y en menor grado con S.
patens.
M. demissus presenta una tasa
baja de crecimiento en el área estudiada (0.8 mm/mes, 0.2 g peso
seco/mes). La mortalidad fue alta para los moluscos suspendidos en el agua. El
crecimiento fue alto durante los meses calurosos. La tasa de respiración
de M. demissus se relaciona al tamaño, a la
temperatura y a la velocidad de la corriente.
El consumo promedio de oxígeno para la talla
media del molusco a 25 ± 0.5°C y salinidades de 16 a 30 %o fue 1.07
± 0.18 y 1.30 ± 1.16 mg 02/g peso seco hora, respectivamente. La
tasa para ejemplares mayores fue 0.72 ± 0.11 y 0.50 ± 0.22 mg
02/g peso seco hora, respectivamente. Los cálculos basados sobre los
datos de la colección indican un presupuesto de carbono orgánico
de 43.4 g/m² año de asimilación, 32.8 de respiración
y 10.6 de producción secundaria.
An ecological study of Modiolus
demissus was carried out in Louisiana coastal marshes from September
1973 until May 1974. The average biomass in the study arca was 6.5 g dry wt
m-² ranging from 49.9 g m-² to 0.5 g m-². Total biomass was
related more to the size of mussels than to total number. The mussels form
small colonies 0.2 to 0.3 m in diameter. The average density was 27.8
individuals m-². There was no preferred siphon orientation. M.
demissus was found from the Gulf shore to the inland extent of the
brackish marsh. The mean annual salinity in these areas ranges from 29.4 ppt to
4.3 ppt. They were not found in the intermediate marshes. Mussels in the saline
marsh were associated primarily with Distichlis spicata and
Spartina patens. In the brackish marsh they were associated
with Juncus roemerianus and to a lesser degree with S.
patens.
M. demissus had a low rate of
growth in the study arca (0.8 mm/mo, 0.2 g dry wt mo-¹). Mortality was
higher for mussels suspended in the water. Growth was higher during the warmer
months. The respiration rate of M. demissus is related to
size temperature and current speed. The average oxygen consumption for medium
size mussels at 25 ± .5°C and salinities of 16 and 30 ppt were 1.07
± 0.18 and 1.30 ±: 1. 16 mg 02 [g dry wt hr] -¹,
respectively. The rates for large mussels were 0.72 ±: 0. 11 and 0.50
± 0.22 mg 02 [g dry wt hr] -¹, respectively. Calculations based on
collected data indicate an organic carbon budget of 43.4 g m-² yr
assimilation, 32.8 respiration, and 10.6 secondary production.
The mussel Modiolus demissus granossissimus Sowerby is an important component of salt marsh macrofauna (Kuenzler, 196la; Lent, 1967; and Day et al., 1973). High tolerance of dehydration and enzymatic thermostability protect this mussel from desiccation, variation in salinity, thermal stress and possible anaerobic conditions. The above cited factors plus air-gaping are responsible for vertical penetration up to mean high water (Dexter, 1947). M. demissus is a filter feeder. Food items include dinoflagellates, small diatoms, bacteria, zoospores, minute ova and spermatozoa, flagellates, and other protozoa as well as detritus consisting of particles from the cystolysed cells of a great variety of plants and animals (Coe et al., 1942). It is also important as a geochemical agent. Kuenzler (1951b) showed mussels can remove 5.4 rng Pm-² of particulated phosphorus daily from the water of which 0.78 mg Pm-² is used as food and 4.7 mg Pm-² is deposited on the marsh surface as pseudofeces where it can be more readily used by marsh plants. The Gulf ribbed mussel Modiolus demissus granossissimus Sowerby, has a distributional range along the east coast of Florida and in the Gulf of Mexico from Florida to the Yucatán Peninsular (Mexico). It is differentiated from the Atlantic ribbed mussel Modiolus demissus Dillwyn, which ranges from the Gulf of St. Laurence to South Carolina, because it has almost twice as many ribs which are more finely beaded (Abbot, 1954). M. demissus in Louisiana was studied as part of an analysis of community structure of the salt marsh (Day et al., 1973). Because this earlier work indicated that M. demissus was an important component of the marsh fauna, a more detailed study of this important dweller of the marsh and its interaction with its habitat was considered appropriate. The purpose of this study was to measure the distribution, biomass, growth and respiration of M. demissus. STUDY AREAThis study was carried out in brackish and saline marshes bordering Barataria and Caminada bays in southern. Louisiana (Fig. l). The dominant vegetation in the brackish marsh is Spartina patens; in the saline marsh, S. alterniflora. Most of the work was conducted in saline marshes surrounding Airplane Lake and in the lake itself. The area is characterized by generally warm temperatures (mean annual water temperature about 20°C) and a relatively low diurnal tide range (~ 0.3 m). Salinity in the study arca ranges from 15 to 26 ppt. Detailed descriptions of the arca are given by Kirby and Gosselink (1976), Day et al. (1973), and Happ et al. (1977). MATERIALS AND METHODSSeveral aspects of mussel ecology were studied. These included biomass distribution, orientation, range, growth, and respiration. BIOMASS DISTRIBUTIONThe study of biomass distribution was carried out in the marshes surrounding Airplane Lake. Seven transects were established and samples were taken from 0.25 m² quadrats at the shore, 3, 5, 10, 20, 30 and 50 meters into the marsh. In the field mussels were washed and fixed in 10% formalin. No samples were taken farther inland because in an earlier work in the area (Day et al., 1973) no mussels were found beyond 50 meters. 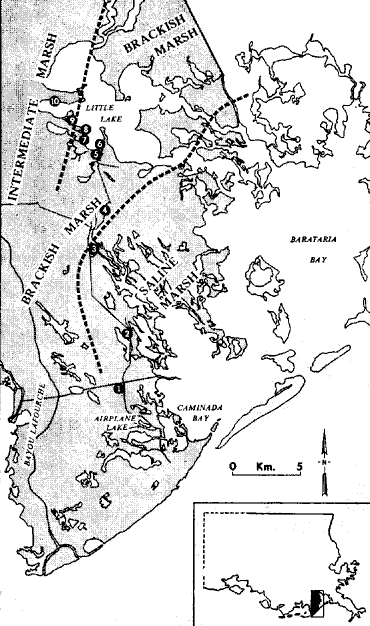 Fig. 1. Map of the southwestern area of Barataria Bay showing the location of the range station and Airplane Lake where intensive studies were carried out. In the laboratory the mussels were washed, and the shell length of each mussel was measured to the nearest millimeter with a Vernie caliper. The meat and the shell were separated, the meat blotted dry and wet meat weight obtained. The meat and shell from each mussel were then separately dried at 105° C for three days to obtain individual dry weight. The dry meat and shells were then fired at 450° C for hours to obtain ash free dry wieght (organic matter). Shell length and meat weight data were used to construct a length-weight diagram (Fig. 2). ORIENTATIONThe orientation and position of all mussels in three 25 m² marsh plots near Airplane Lake located at shore, 50, and 100 meters were determined. The plots were divided by sticks and strings into one m² areas. All plants were carefully removed from each square meter and the number and orientation of each musel were recorded. The orientation was determined by the direction of the syphon. RANGEThe determinatlon of the range of M. demissus in Louisiana was done along a transect in the southwest region of Barataria Bay in May 1974 (Figure l). Ten sampling sites were chosen in saline, brackish, and intermediate marshes. The marsh classification of saline, brackish, and intermediate was based on the plants present at the different sites as described by Chabreck (1972). Stations 1, 2, and 3 were located in saline marsh; stations 4, 5, 6, and 7 in brackish marsh; station 8 was located at the transition zone between brackish and intermediate marsh; and stations 9 and 10 were located in intermediate marsh. Each station was checked for the presence or absence of mussels along the shore and a few meters inland. Salinity was recorded and dominant plants at each site were identified. GROWTH RATEMussel growth rate was studied from September 1973 until May 1974. About 100 small mussels (mean shell length 33.5 mm) were collected from the saline marsh. The mussels were separated, cleaned, and the shell length (from apex to posterior end) measured to the nearest millimeter. Two groups of 40 individuals each were placed in 50 X 30 X 30 cm wire cages with 7 mm mesh. 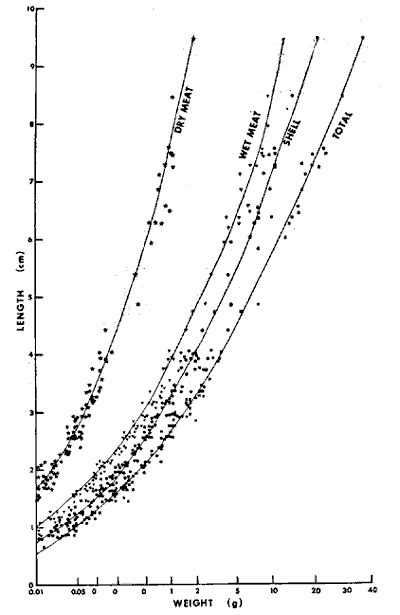 Fig. 2. Length-weight diagram for Modiolus demissus. In order to observe the effect of the habitat on growth rate the cages were placed in two locations in and around Airplane Lake. One was placed in the Spartina marsh approximately 10 meters from shore and fixed so the mussels were level with the surface of the marsh. The second cage was placed at the mouth of a small tidal creek. The latter cage was fixed with floats so that it was suspended just under the water. Monthly the cages were pulled, the mussels were removed, cleaned of fouling organisms, and measured. Normally most of the mussels were entangled by the byssus threads. To facilitate measurement they were separated by cutting the byssus threads. After measuring, they were put back in the cages, which were cleaned of fouling organisms, and returned to their respective locations. A mean length and standard error was calculated for each cage and sampling time. The length-weight diagram (Fig. 2) was used to convert increase in shell length to increase in weight. RESPIRATIONA closed, continuously-flowing, recording differential respirometer was used to measure the oxygen consumption of the mussels. Basically it consists of a test chamber positioned between two polarographic oxygn electrodes through which a known volume of water flows from a supply to a catchment reservoir. The respirometer is described in detail by Bishop (1974) and Rodríguez-Ortega (1974). The mussels used in the respiration experiment were collected by hand February 5, 1974, from the marsh around Airplane Lake. In the laboratory the mussels were kept at 20 ppt sahnity and at a temperature of 25° C. The water for the aquarium and the experiment itself was made using tap water and Instant Ocean. Two groups of mussels were chosen for respiration measurements; one group of large mussels (76.8 mm average length) and one group of small mussels (48.7 mm average length). The shells were thorougholy cleaned. The mussels were acclimated to the respirometer conditions (salinity and temperature) for at least 48 hours, during which time no food was provided. Oxygen consumption was measured at 25 ± 0.5°C and at salinities of 16 and 30 ppt. After the probes were synchronized, a mussel was placed in the chamber, the chamber voided of air and sealed, and the water switched to flow through the test chamber. Dissolved oxygen concentration before and after flowing through the chamber was constantly recorded. At the end of a test, the water flow was again switched from probe to probe to check if both probes were reading equally. After the test the shell length was measured and total wet body weight and dry meat weight were obtained as described earlier. All tests lasted one hour. The first 30 minutes allowed the mussel to partially acclimate to flowing water conditions. The data from the last 30 minutes were analyzed for respiration. Nine replicates were run for each test case. Respiration was calculated by using the difference of the oxygen from the incoming and outgoing water (mg O2/ml), the total amount of water that had passed through the chamber during the experiment (ml/hr), and the dry meat weight. The following equation was used to calculate oxygen consumption (in mg 02-g dry body wt-¹.hr-¹) : O2, difference [ _mg0²/ml ] x flow rate [ ml/hr ] ÷ dry w t (g) = mg 02 [g dry wt - hr] - ¹ RESULTS
BIOMASS DISTRIBUTIONThe highest number of mussels was collected in the marsh near shore (Fig. 3b), however the highest biomass was collected 50 meters from the shore (Fig. 3e). This is because individuals were larger at the 30 and 50 meter stations (Fig. 3a, c, d) . The average dry biomass in the study area (shore to 50 meters) was 6.45 g m-² and the average density was 27.8 individualsm m-². The highest single biomass 49.9 g m-² and the lowest was 0.5 g m-² indicating the clumped nature of mussell distribution. ORIENTATIONThe resulsts of the mapping study show the clumped pattern of distribution (Fig. 4). Some mussels were isolated but most of them occurred in clumps, especially near the shore. The size of the clumps was generally between 0.2 and 0.3 m in diameter. No specific orientation was found in any of the three plots. The average number of mussels per square meter for plots 1, 2, and 3 was 7, 6, and 14, respectively. RANGEM. demissus was found at all saline and brackish marsh stations. None were collected in intermediate or fresh marsh (Table 1). Measured salinities ranged from 11 to 0 ppt. The mean salinity for saline marsh is 18.3 ppt (range 8.1 to 29.4 ppt); and for brackish marsh, 9.9 ppt (4.3 to 18.4 ppt) (Chabreck 1970). GROWTHM. demissus had a low Yate of growth (0.8 mm/mo; Fig. 5) With the exception of the first month (marsh 3.6 mm/mo; water-2.8 mm/mo), the mussels suspended in the water grew slightly faster than those in the marsh; however this difference was not statistically significant. Intial mean shell length of all mussels used was 33.5 mm. From the length-weight diagram (Fig.2) we calculated that the average weight gainfor mussels was 0.06 g and 0.08 g dry wt/g mus sel/month for marsh and water, respectively. The mussels and the cages had to be periodically cleaned of fouling organisms such as barnacles, bryozoans, and filamentous algae which heavily covered the mussels and the walls of the cage suspended in the water. We estimate that 10 to 20% of the water circulation was blocked. The mussels in the Spartina marsh were covered by only a few fouling organisms. Fouling was a problem during spring and summer and disappeared in winter. Higher mortality was observed for the mussels suspended in the water and during warmer months. For mussels in the water the mortality was 7.5%, from. September to November, 0%, from December to March, and 56.7% mortality between April and May. For the marsh mussels mortality for the same, intervals was 2.5%, 0%, and 15.3%, respectively. Since the mussels were protected from predation, the values obtained should represent natural mortality without predation. RESPIRATIONSmall size mussels had a higher rate of oxygen consumption per gram than larger ones but salinity did not produce a definite effect (Table 2). The average rate of oxygen consumption of smaller mussels (48.7 mm) was 1.07 and 1.30 mg 02 (g dry wt.hr)-¹ at 16 and 30 ppt, respectively. For larger mussels (76.8 mm) comparable rates were 0.72 and 0.50, respectively. 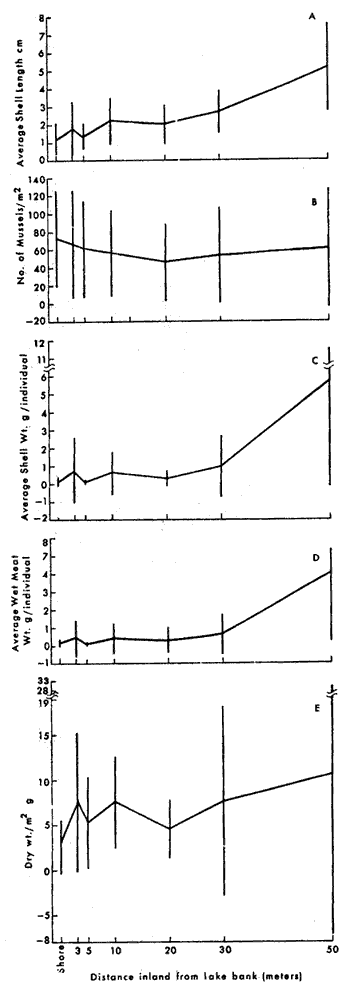 Fig. 3. Results of transects showin average shell lenght (A), number of mussels m-2 (B), mean individual shell weight (C), individual wet weight of mussel meat (D), and total biomas (E), Vertical bars are standar deviation. DISCUSSION
DISTRIBUTIONThe average biomass found in this study (6.45 g dry wt m-²) is higher than the value found in an earlier work, 3.19 g m-², carried out in the same area by Day et al., (1973). The average biomass found in Georgia for M. demissus was 4.55 g m-² (Kuenzler 196la). There were no differences in the shell/meat ratio for the different stations. Similar findings were reported by Lent (1967) for mussels in Delaware. In their study of marsh macrofauna at Airplane Lake, Day et al. (1973) defined a marsh edge zone where most animal biomass occurred. They found the highest density of M. demissus near the shore; the single highest biomass occurred at 3 meters (9.5 g m -²). In this study we found the highest average biomass at 50 meters, where the single highest biomass collected was 49.8 g m-². The difference in these two findings probably reflects a difference in the nature of the marsh. The area where Day et al., sampled is a uniform marsh. The area where we sampled is laced with many tide channels and pools. Therefore our failure to observe any pronounced "edge effect" is probably because the entire area sampled could be characterized ecologically as edge habitat. The mapping results clearly (Fig. 4) show a clumped pattern of distribution, but neither individual mussels nor the clumps show any pattern of orientation towards bodies of water or direction of currents. This is probably because the water moves so slowly through the marsh with each tide without the development of swift currents which may be the requirement for mussels to develop a definite orientation. The size of the clumps is also related to water exchange. The development of mussel beds is related to currents produced during tidal exchange, rather than wave action. Large colonies develop along coasts with a large tidal range. Examples are the large beds of Mytilus edulison the Atlantic coast and M. demissus in Georgia where clumps 1 meter in diameter, and several meters long are found parallel to the banks of tidal creeks (Kuenzler 196la). In Louisiana the tide range is about 0.3 m. High currents occur only in such arcas as tidal passes. Currents are normally sluggish in bayous and lakes and water movement occurs as sheet flow through the marsh grass. Because of this, the amount of water exchange through a particular section of marsh surface is not enough to support a large colony. 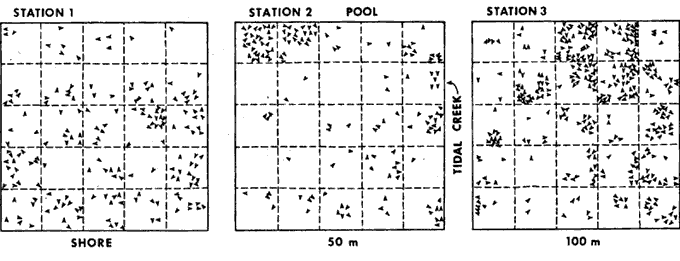 Fig. 4. Number and orientation of mussels in three 25 m2 marsh plots, Size of small divisions is 1 m2. RANGEMussels were found at all saline and brackish marsh stations (1 to 7), but were absent from intermediate marsh stations (8 to 10) (Table 1). The mean annual salinity of arcas where mussels were present ranges from 29.4 ppt in the saline marsh to 4.3 ppt in the brackish marsh (Chabreck 1972). Sedney (1971) found that the subspecies Modiolus demissus Dillwyn on the Atlantic coast had a salinity range of 3 to 36 ppt. Based on the salinity readings at the time of this study, it is our opinion that M. demissus granossissimus can withstand exposure to fresh water for short periods of time. Mussels tended to form clumps around plants which develop thicker root mats. In the saline marsh where S. alterniflora, S. patens, and D. spicata are present, mussels were found preferentially associated with S. patens and D. spicata. In the brackish marsh mussels were found preferentially associated with J. roemerianus and to a lesser degree with S. patens.  TABLA 1 DESCRIPTION OF THE STATIONS SAMPLED FOR DETERMINATION OF THE RANGE AF MODIOLUS DEMISSUS IN LOUSIANA 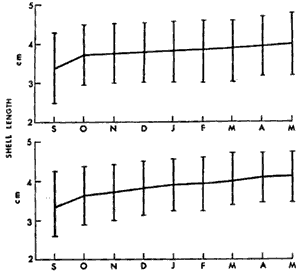 Fig. 5. Growht of the mussels in the marsh (top) and in the water (bottom). Vertical bars are standard deviation. GROWTHThe growth values obtained in this experiment were low (0.8 to 0.9 mm/month) when compared to the growth of the Atlantic ribbed mussel M. demissus Dillwyn, of 2 mm/mo (Kuenzler 196la) and for Mytilus edulis in Europe of 5 mm/mo (Bardach 1972). The low growth rate found here may be related to three factors. First, this experiment was from Septem, ber to May, through the cooler months when the growth rate probably decreases. Second, the byssus threads were cut cach month to facilitate measurement and the mussels had to expend some energy for growth of new threads. Third, the low tidal range produces very sluggish currents in the marsh which may limit suspended particulate matter on which musseles feed. 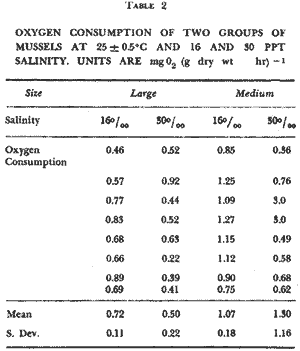 TABLA 2 OXYGEN CONSUMPTION OF TWO GROUPS OF MUSSEELS AT 25+-0.5°C AND 30 PPT SALINITY. UNITS ARE mg02 (dry wt . hr) -1 RESPIRATIONRespiration values obtained in this study compare favorably with those reported by Read (1962) who also measured oxygen consumption of individual mussels. He found 1.18 cc 02 g-¹ hr-¹ for large mussels as compared to our measurements of 1.07 mg 02 g-¹ hr-¹ for medum size mussels and 0.72 for large mussels. Read also found that the metabolic activity of Mytilus edulis reached a maximum around 20° C while that of Brachiodontes demissus plicatulus increased up to 35.2° C. Mytilus edulis had a higher rate of respiration at low temperatures. It is likely that M. demissus is similar to B. demissus. Salinity seemed to have no significant effect on respiration. CARBON BUDGETUsing the data generated in this study an annual carbon budget of the mussel population in the Airplane Lake marsh was calculated (Fig. 6). For respiration, an hourly rate of 0.90 mg 02 g dry wt-¹ was used. This is the average rate for large and small mussels and is equivalent to 50.9 g 02 m-² yr-¹. This value was converted to carbon metabolism using the atomic ratios for the metabolism of glucose, where 32 g 02 are used to metabolize 12 g of carbon. Therefore, 50.9 g 02 is used to burn 32.8 g organic matter. For the growth calculation we used the average growth rate of individual mussels on the marsh (0.17 g dry wt mo-¹ or 2.04 g dry wt yr-¹), The average mussel was 38.2 mm and weighed 1.24 g dry wt (from Fig. 2). Thus there are 2.04 g dry wt growth for each 1.24 g dry wt of mussel. Since the average mussel biomass was 6.45, the total calculated growth is 10.6 g dry wt-² yr-¹. These data are for the marsh zone within 50 meters of shore. For the same area of marsh, Day et al., (1973) used literature values to estimate a respiratory rate of 64 g dry wt m-² yr-¹ and a growth rate of 6.4 g m-²hr-¹. This budget is for assimilated material which is used respiration or secondary production; there is no consideration of fecal or pseudofecal production. 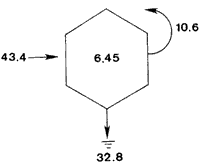 Fig 6. Carbon budget of M. demissus at Airplane Lake marsh (shore to 50 meters). 6.45 is mean annual biomass in g dry wt m-2. The other numbers are flows in g dry wy m-2 yr-1 for assimilated carbon (43.4), respiration (32.8)m, and secondary production (10.6). AgradecimientosThis study was supported by the Louisiana Sea Grant Program, a part of the National Sea Grant Program maintained by the National Oceanic and Atmospheric Administration of the Dept. of Commerce. Support was also provided by Louisiana Offshore Oil Port, Inc. LITERATURAABBOTT, R. T., Princeton, N. J.: D. Van Nostrand Co., American Seashells. 1954 351-352 BARDACH, J. E., J. H. RYTHER, and W. O. Mc LARNEY, The Farming and Husbandry of Fresh and Marine Organisms. Aquaculture. 1972 868 BISHOP, J., The influence of selected conditions on the oxygen consumption of the brown shrimp, Penaeus aztecus, in a closed flowing differential respirometer. La. State Univ., Baton Rouge. Ph. D. dissertation 1974 CHABRECK, R. H., Marsh zone and vegetative types in the Louisiana Coastal Marshes. Ph. D. dissertation, La. State Univ., Baton Rouge. 1970 113 Vegetation, Water and Soil Characteristics of the Louisiana Coastal Region. Louisiana State University Agricultural Experiment Station, Bulletin. 1972 1-72 644 COE, W. R. and D. L. Fox, . J. Exp. Zool., Biology of the California sea mussel (Mytilus californianus) Influence of temperature, food supply, sex and age on the rate of growth. 1942 1-30 90 DAY, J. W., W. G. SMITH, P. R. WAGNER, and W. STOWE, Community Structure and Carbon budget of a salt marsh and shallow bay estuarine system in Louisiana. Publ. N° LSU-SG-72-04 1973 79 DEXTER, R. W., Ecol. Monogr., The marine communities of a tidal inlet at Cape Ann, Massachusetts: A study in bio-ecology. 1947 261-294 17 3 HAPP, G., J. GOSSELINK, and J. DAY, Estuar. Coast. Mar. Sci., The seasonal distribution of organic carbon in a Louisiana estuary. 1977 695-705 5 KIRBY, J. and J. GOSSELINK, Ecology Primary production in a Louisiana gulf coast Spartina alterniflora Marsh. 1976 1052-1059 57 2 KUENZLER, E. J., Limnol. Oceanogr., Structure and energy flow of a mussel population in a Georgia salt marsh. 1961a 191-204 6 Limnol. Oceanogr., Phosphorus budget of a mussel population. 1961b 400-415 6 LENT, C. M., Chesapeake Science, Effect of habitat on growth indices in the Ribbed mussel, Modiolus (Arcuatula) demissus. 1967 221-227 8 4 American Zoologist Adaptations of the Ribbed mussel, Modiolus demissus (Dillwyn), to the intertidal habitat. 1969 238-292 9 2 READ, K. R., Comp. Biochem. Physiol., Respiration of the Bivalve molluscs Mytilus edulis L. and Brachidontes demissus Plica tulus Lamark as a function of size and temperature. 1962 89-101 7 RODRÍGUEZ-ORTEGA, D., An ecological study of Modiolus demissus granossissimus Sowerby in the Louisiana coastal marshes, M. S. Thesis, La. Sta te Univ., Baton Rouge 1974 40 SEDNEY, S. K., Comp. Biochem. Physiol., Volume regulation and valve movement by marine mussels. 1971 103-117 39 1A
|

